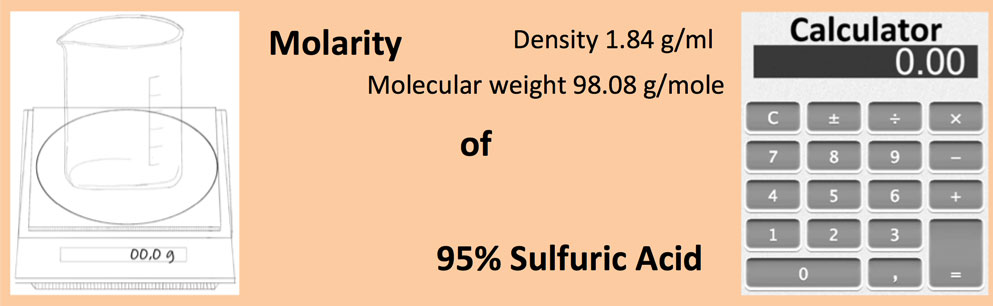
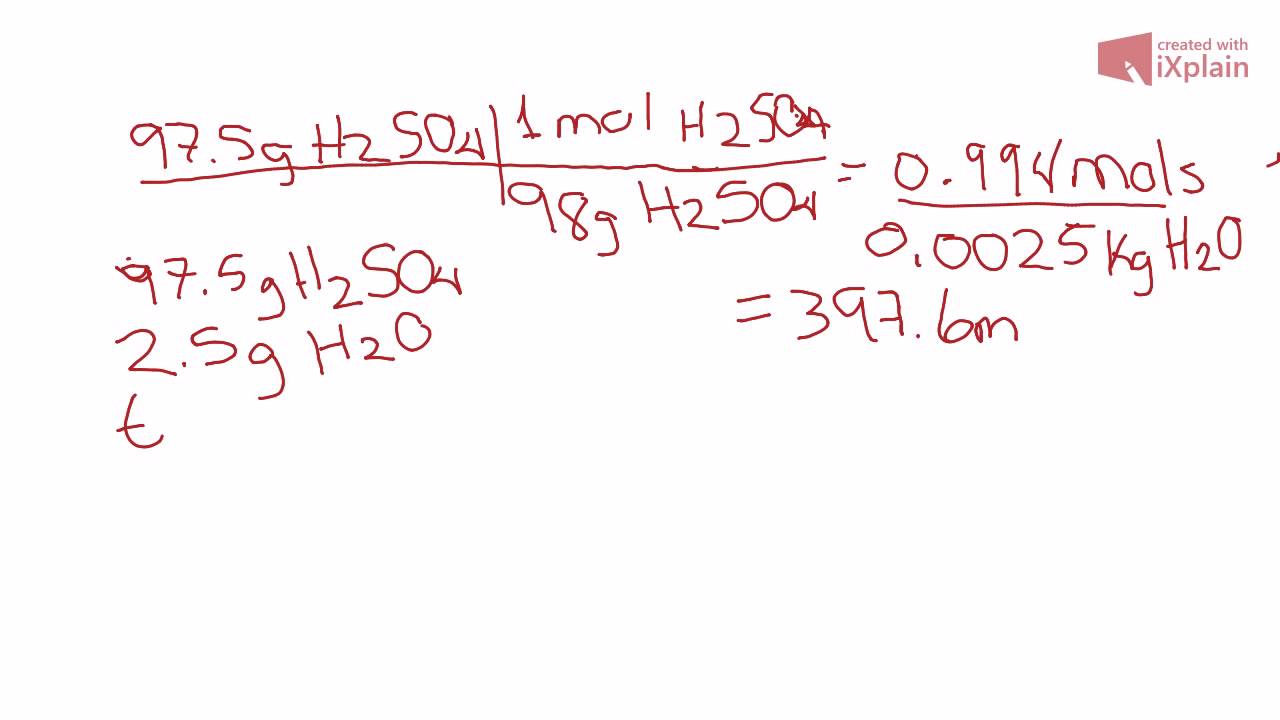What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

Ricca R8215000-500A Sulfuric Acid 500 mL, Poly Natural, 0.04 Normal,0.02 Molarity New Laboratory Setup Savings - up to 40%, Magnetic Stirrer, Vortex Mixer, Sample Prep, Centrifuge

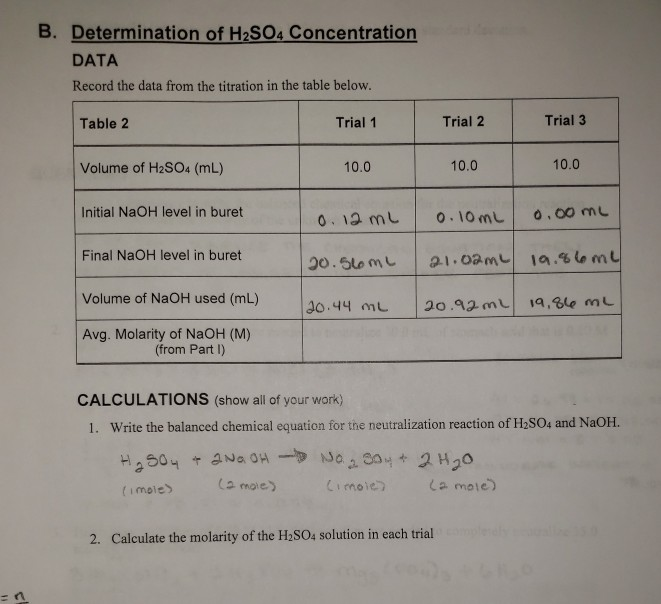
Question Video: Determining the Concentration of Sulfuric Acid Via Titration with Sodium Carbonate | Nagwa

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora
Chem201, Winter 2006 Name Answer key______________ Midterm N1 01/26/06 SID___________________________ 1. A solution is prepared

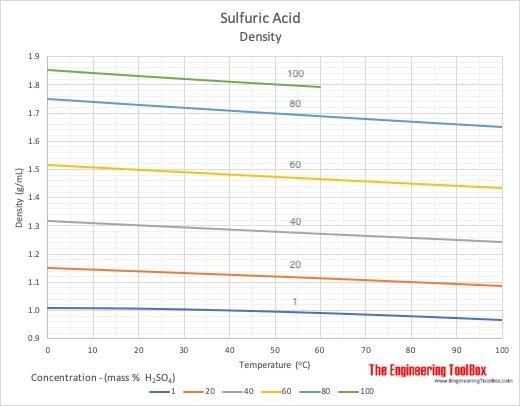
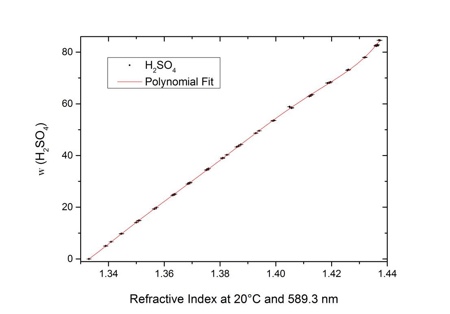
Why does the graph of the electrical conductivity of sulfuric acid/water solutions have this knee in the ~85%-~92% range? - Chemistry Stack Exchange
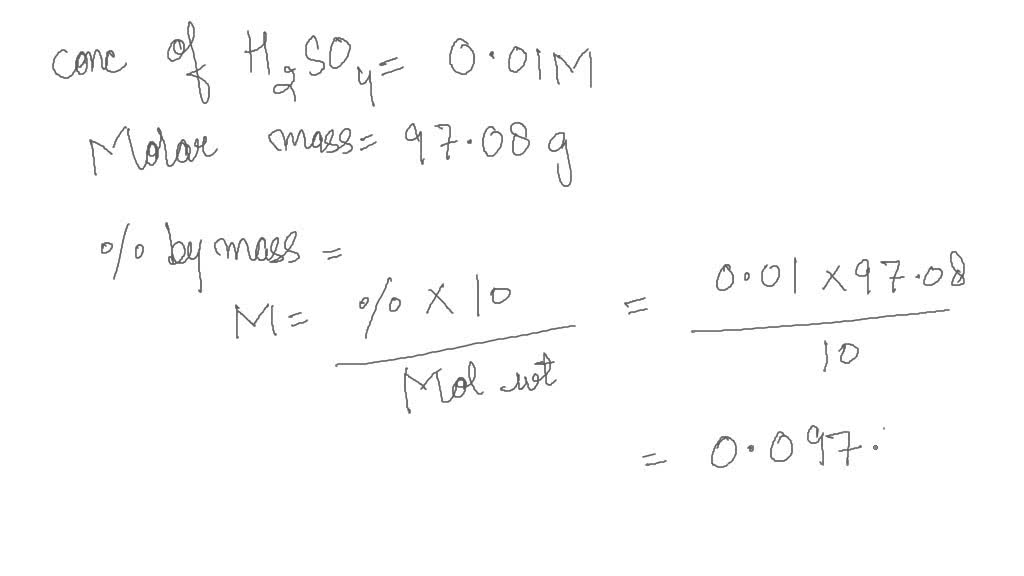
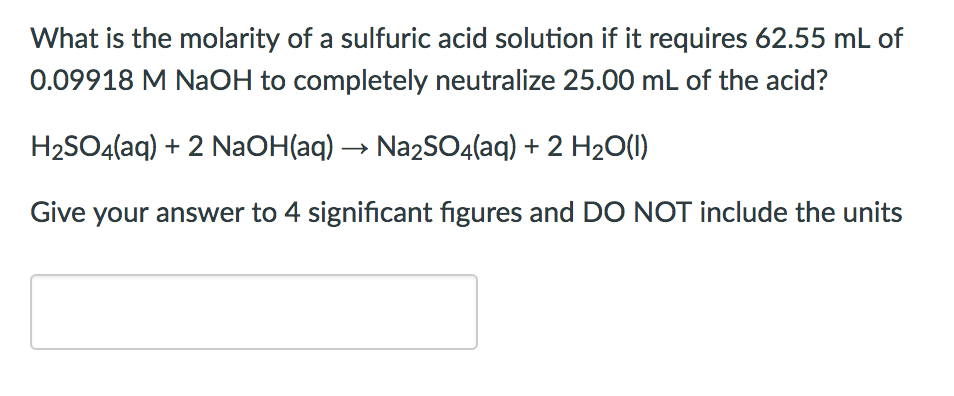
SOLVED: The molarity of a sulfuric acid solution is 0.01 M. Express this concentration in % by mass, ppm, and ppb.(Density of water is 1 g/mL; molar mass of sulfuric acid is
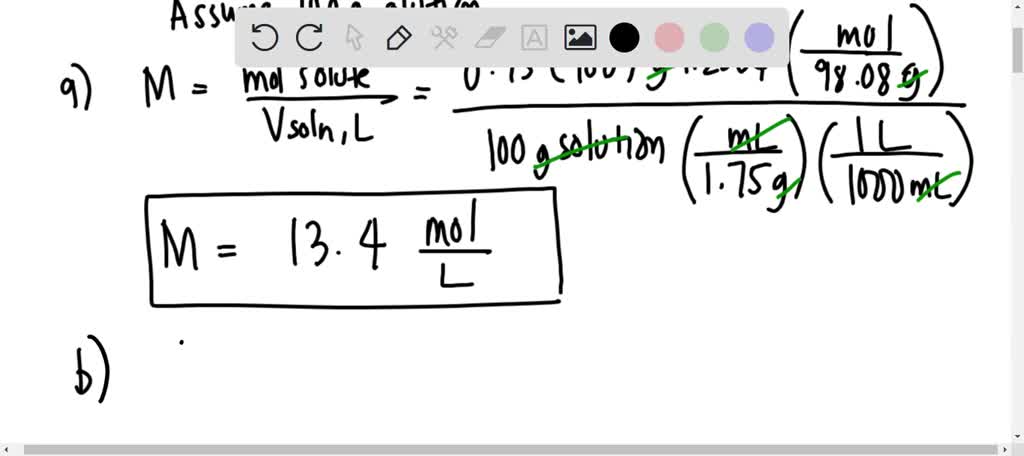
SOLVED: A solution of sulfuric acid contains 75% sulfuric acid by mass. If the density is 1.75 g/ml, Calculate a ) Molarity of the solution and calculate b)mole fraction of the solute

reactivity - How could mass increase when sulfuric acid is added to calcium carbonate? - Chemistry Stack Exchange

The molality and molarity of a solution of sulphuric acid are 4.13 mol/kg and 11.12 mol/ L respectively. Calculate density of the solution.