Brain Sciences | Free Full-Text | Safety and Efficacy of Eculizumab Therapy in Multiple Sclerosis: A Case Series
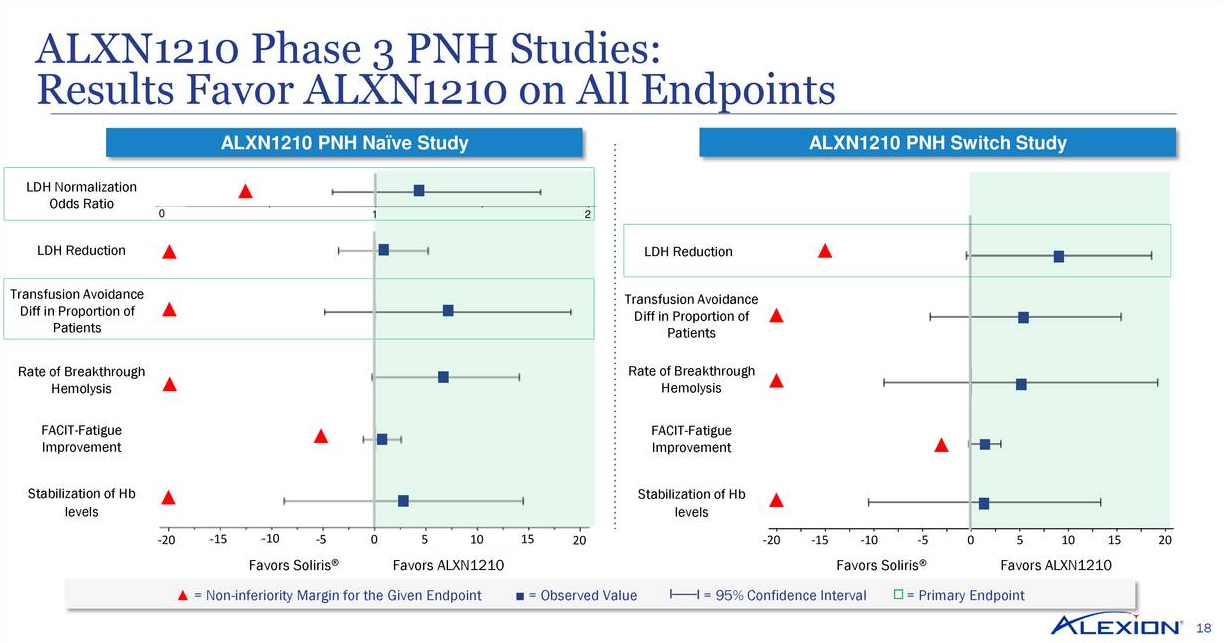
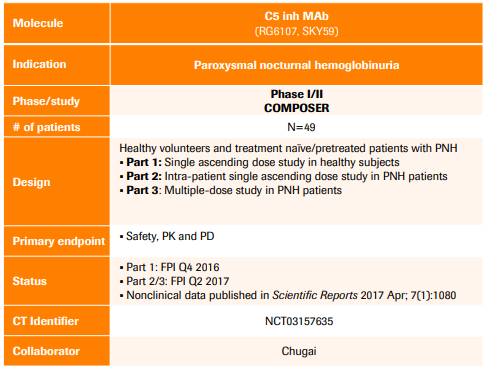
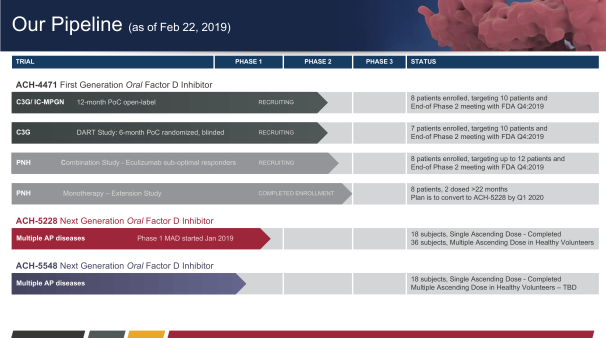
Alexion Pharmaceuticals: Clinical Progress, Acquisitions And The Competition (Part 1) (NASDAQ:ALXN) | Seeking Alpha
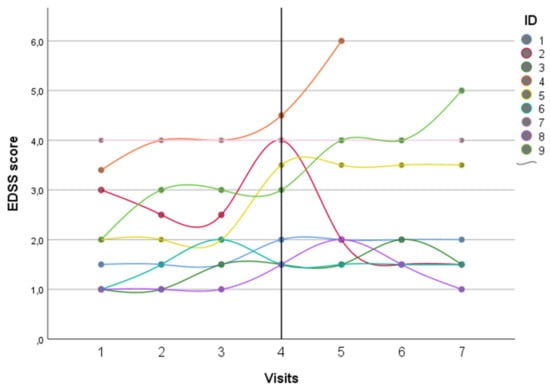
Brain Sciences | Free Full-Text | Safety and Efficacy of Eculizumab Therapy in Multiple Sclerosis: A Case Series
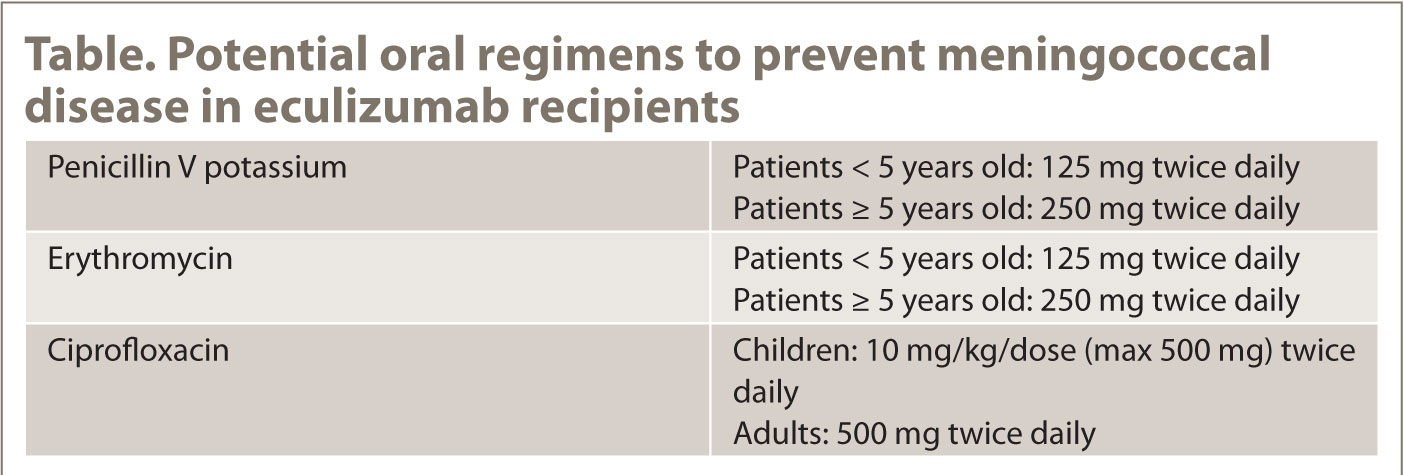
CONCLUSIONS ACKNOWLEDGMENTS DISCLOSURES REFERENCES James F. Howard, Jr,1 Richard J. Nowak,2 Gil I. Wolfe,3 Michael G. Benatar,4