Aqueous hydrochloric acid HCl will react with solid sodium hydroxide NaOH to produce aqueous sodium - Brainly.com

Draw the products of benzoic acid reacting with sodium hydroxide. Draw the products of the pyridine reacting with hydrochloric acid. Use the "+/-" button to add the charge (and H atom).

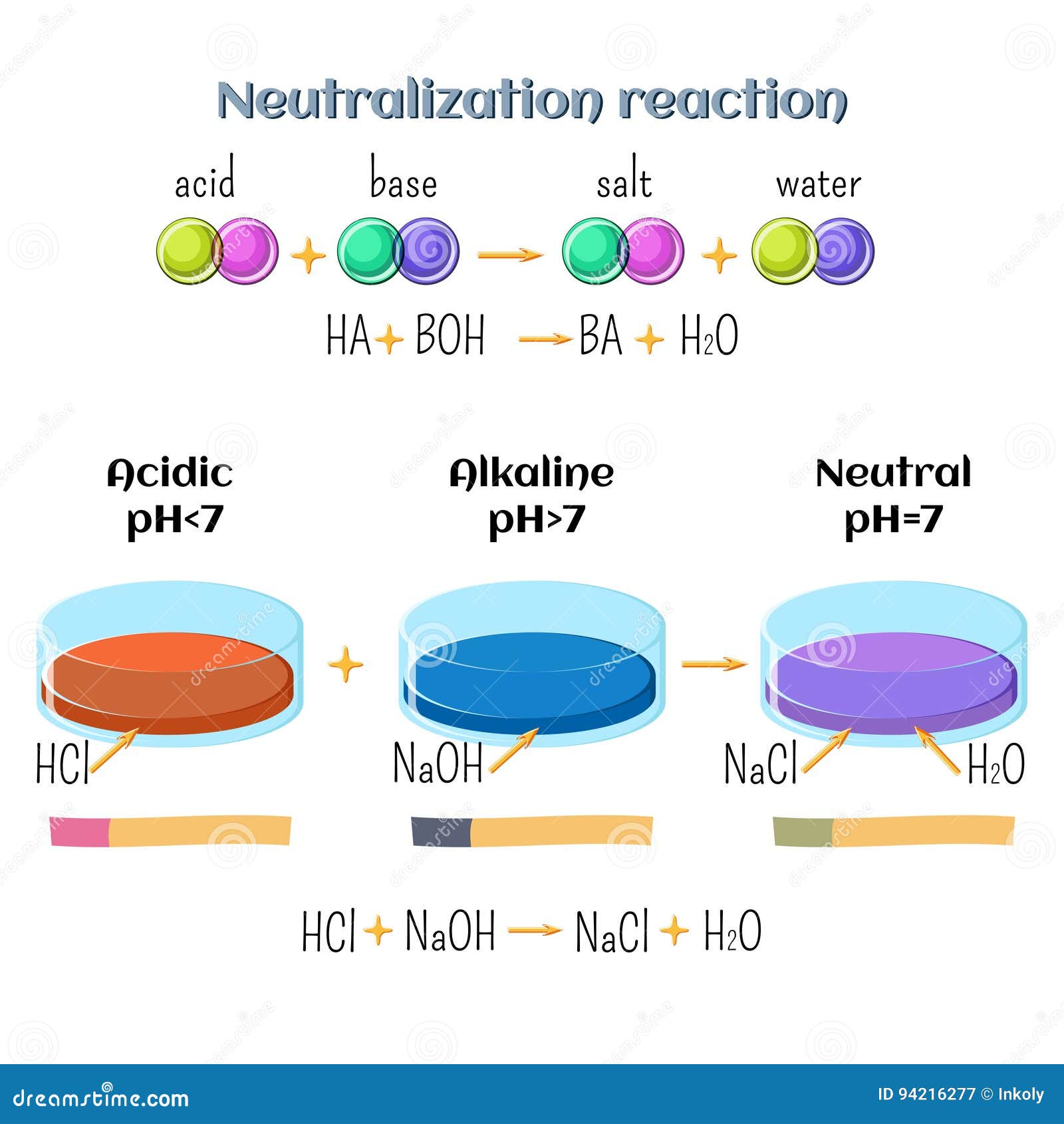
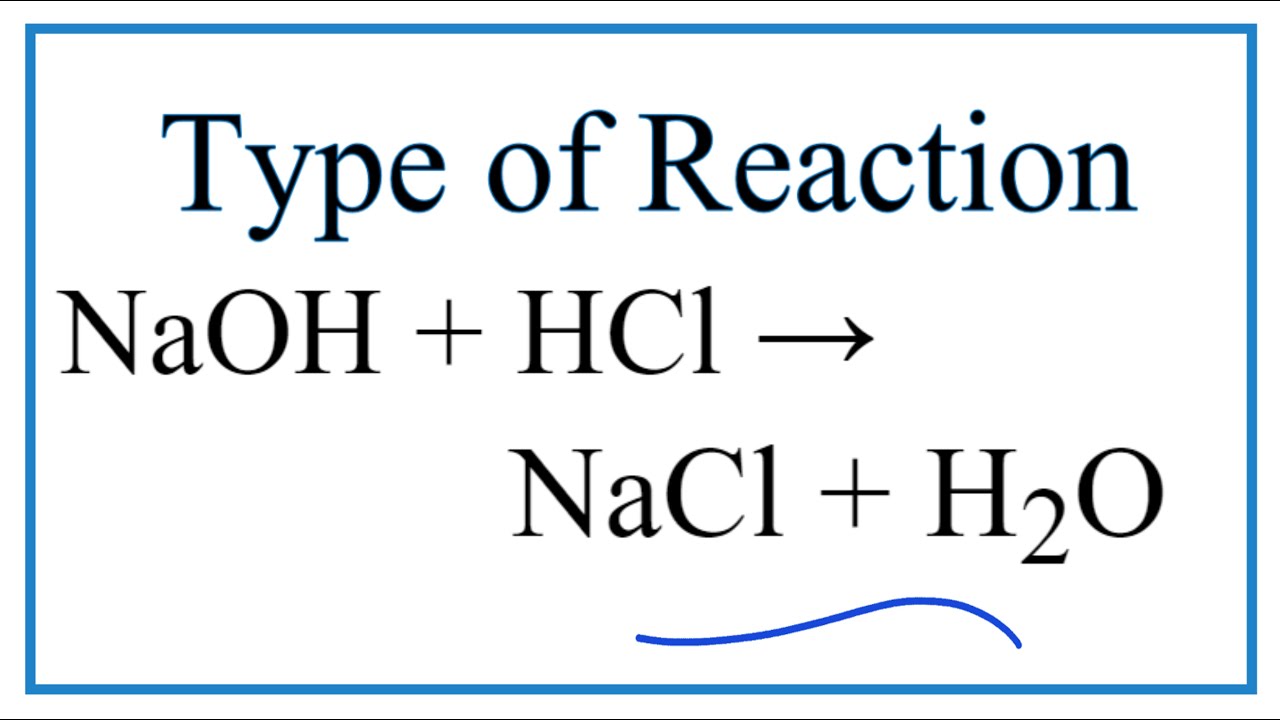
Sodium hydroxide and hydrochloric acid react as shown in this equation. NaOH ((aq))+HCl((aq))toNaCl((aq))+H(2)O((I)) (i)Which type of chemical reaction in this? (ii)The reaction is exothermic .Explain what that means. (iii)Differentiate exothermic ...

Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and write the equation for this process.
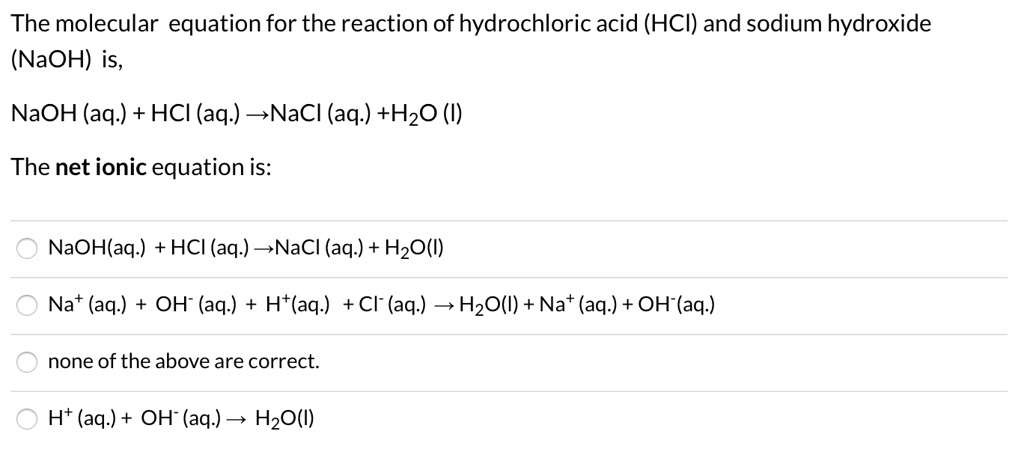
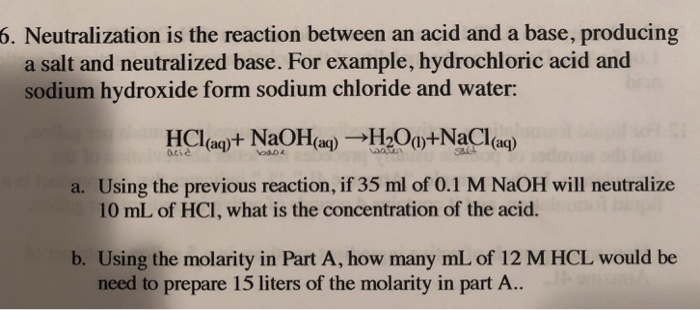
SOLVED: The molecular equation for the reaction of hydrochloric acid (HCI) and sodium hydroxide (NaOH) is, NaOH (aq ) + HCI (aq-) NaCI (aq ) +HzO (I) The net ionic equation is:

Aqueous Reactions Acids There are only seven strong acids: Hydrochloric (HCl) Hydrobromic (HBr) Hydroiodic (HI) Nitric (HNO 3 ) Sulfuric (H 2 SO 4 ) Chloric. - ppt download
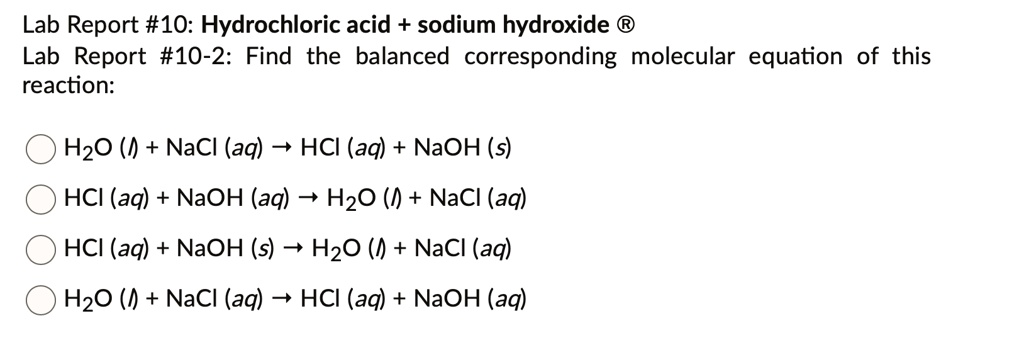
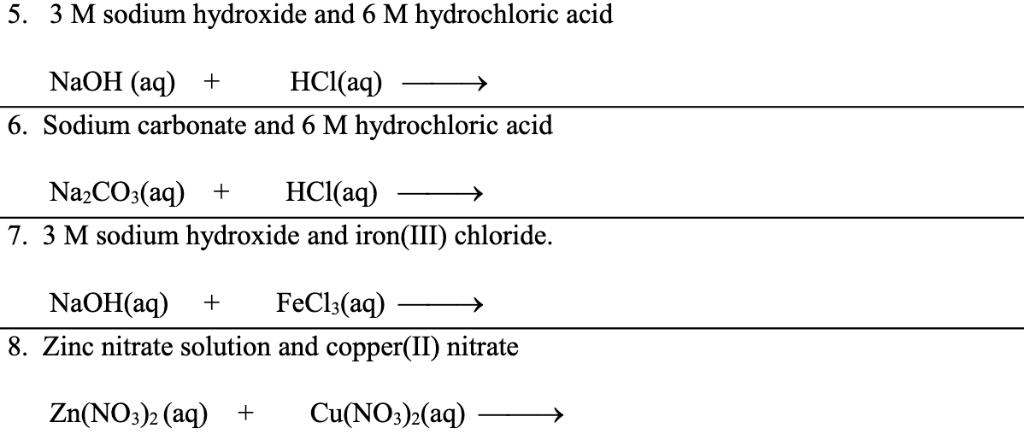
SOLVED: Lab Report #10: Hydrochloric acid + sodium hydroxide Lab Report #10-2: Find the balanced corresponding molecular equation of this reaction: H2O (0 + NaCl (aq) 4 HCI (aq) + NaOH (s)