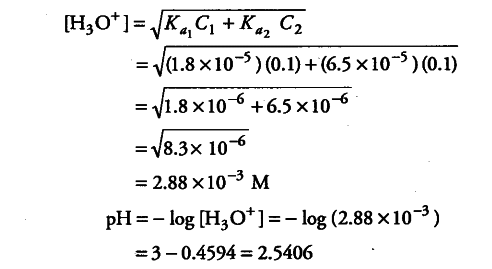What is the pH of 0.1 m of acetic solution, if acetic acid is a weak acid with Ka2 1.86 × 10-5? - Quora

when 10 ml of 0 1 M acetic acid ( pka = 50) titrated against 10 ml of 01 - Chemistry - Equilibrium - 10271133 | Meritnation.com

Calculate the pH of 0.1 M acetic acid solution if its dissociation constant is 1.8 × 10^-5 . If 1 litre of this solution is mixed with 0.05 mole of HCl , what will be pH of the mixture?
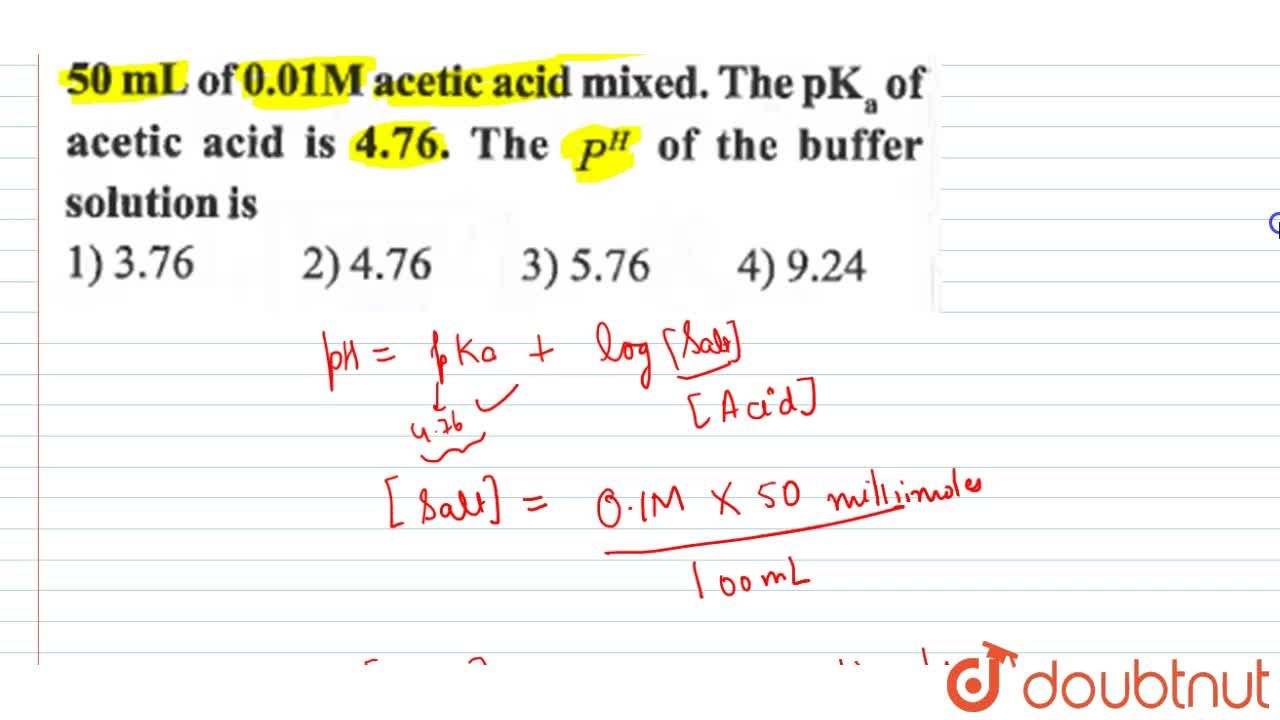
50 mL of 0.1 M solution of sodium acetate and 50 mL of 0.01 M acetic acid mixed. The pK(a) of acetic acid is 4.76. The P^(H) of the buffer solution is
Q22) The pH of 0.1 M acetic acid solution is closest to [Dissociation constant of the acid, Ka=1.8×10^ 5[Mark:4]

What is the tenths digit in the decimal representation of a certain number?(1) The number is less than 1/3 .(2) The number is greater than 1/4

SOLVED: Calculate the pH at the equivalence point when 0.1 M NaOH is titrated with 25 mL of 0.1 M acetic acid.
![The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ] The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/QXVmVDZfQ29GV1k=/sd/)
The pH of 0.1 M acetic acid solution is closest to[Dissociation constant of the acid, Ka = 1.8 × 10^-5 ]