Before the reaction occurs, the concentration of A is 0.071M. If the concentration of A at - Brainly.com
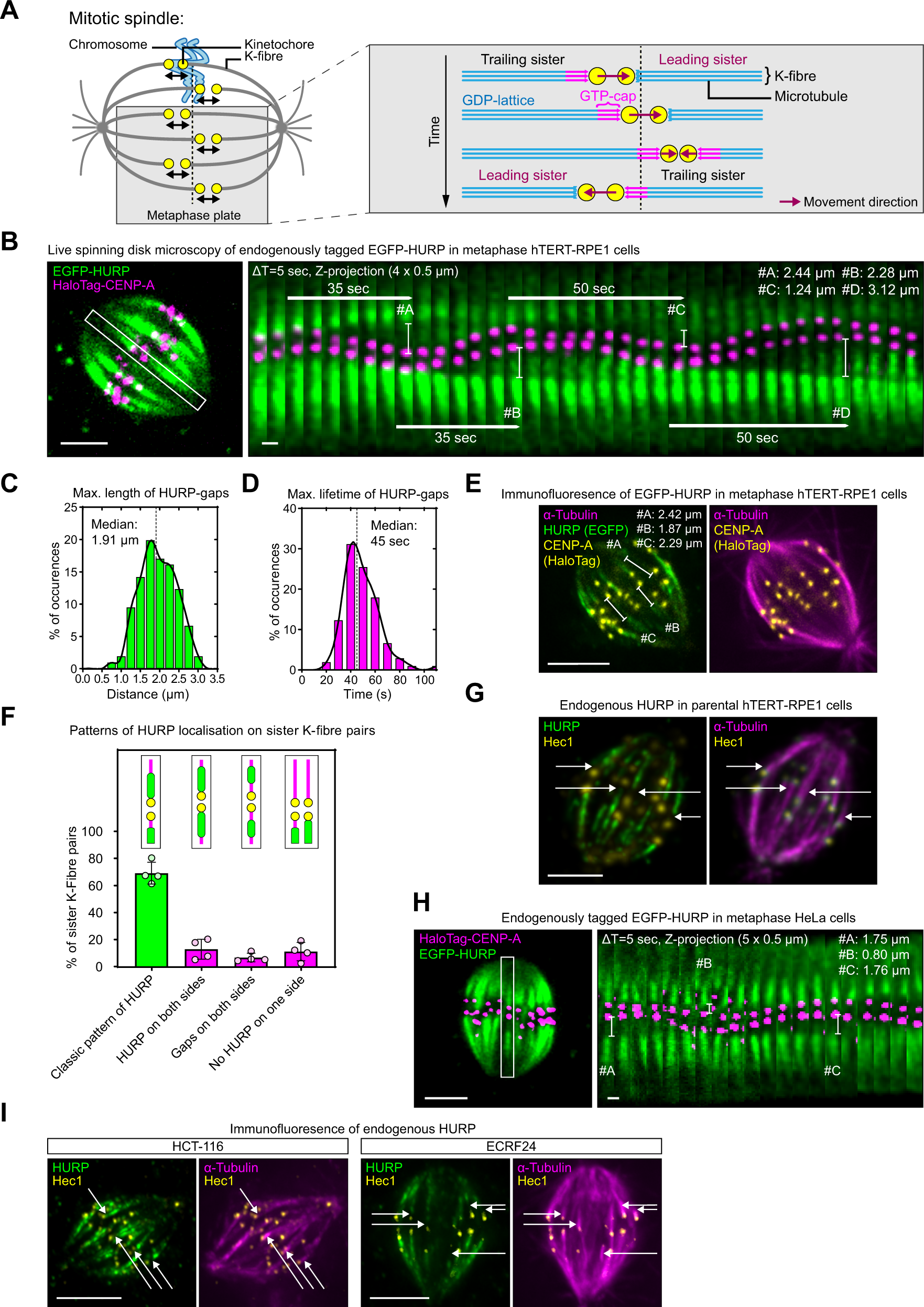
Evidence for a HURP/EB free mixed-nucleotide zone in kinetochore-microtubules | Nature Communications

Consider an endothermic reaction X → Y with the activation energies Eb and Ef for the backward and forward reactions respectively. In general:
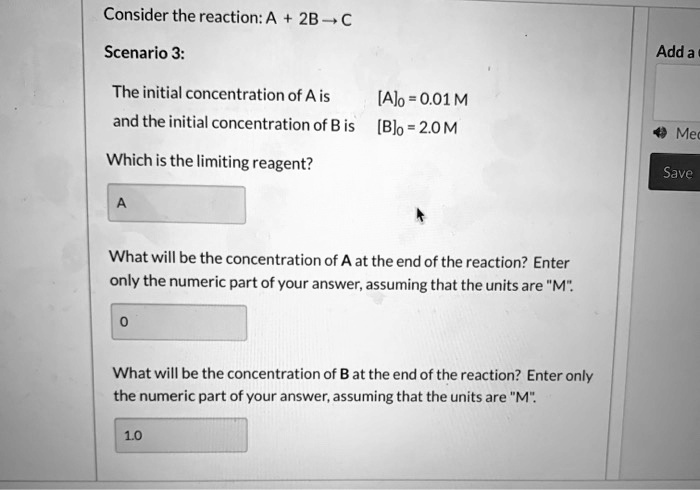
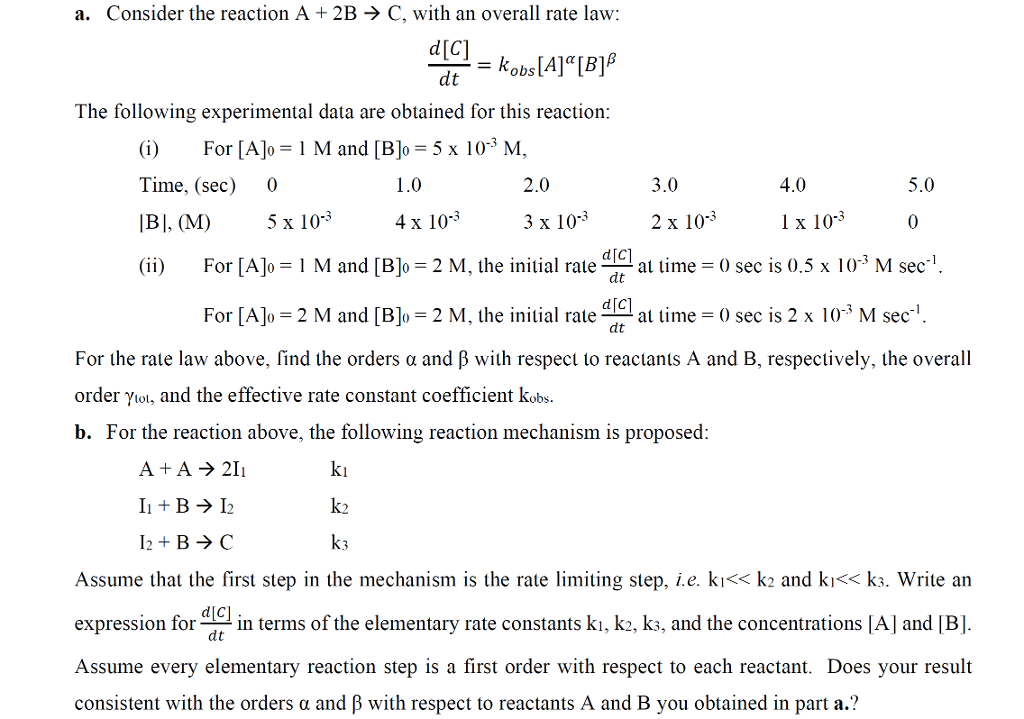
SOLVED: Consider the reaction: A 2B-,C Scenario 3: Adda The initial concentration of A is [Alo = 0.01M and the initial concentration of B is [Blo = 20M Which is the limiting

OneClass: c. Based on your answer to question 2b above and the amount of aluminum metal you used in y...

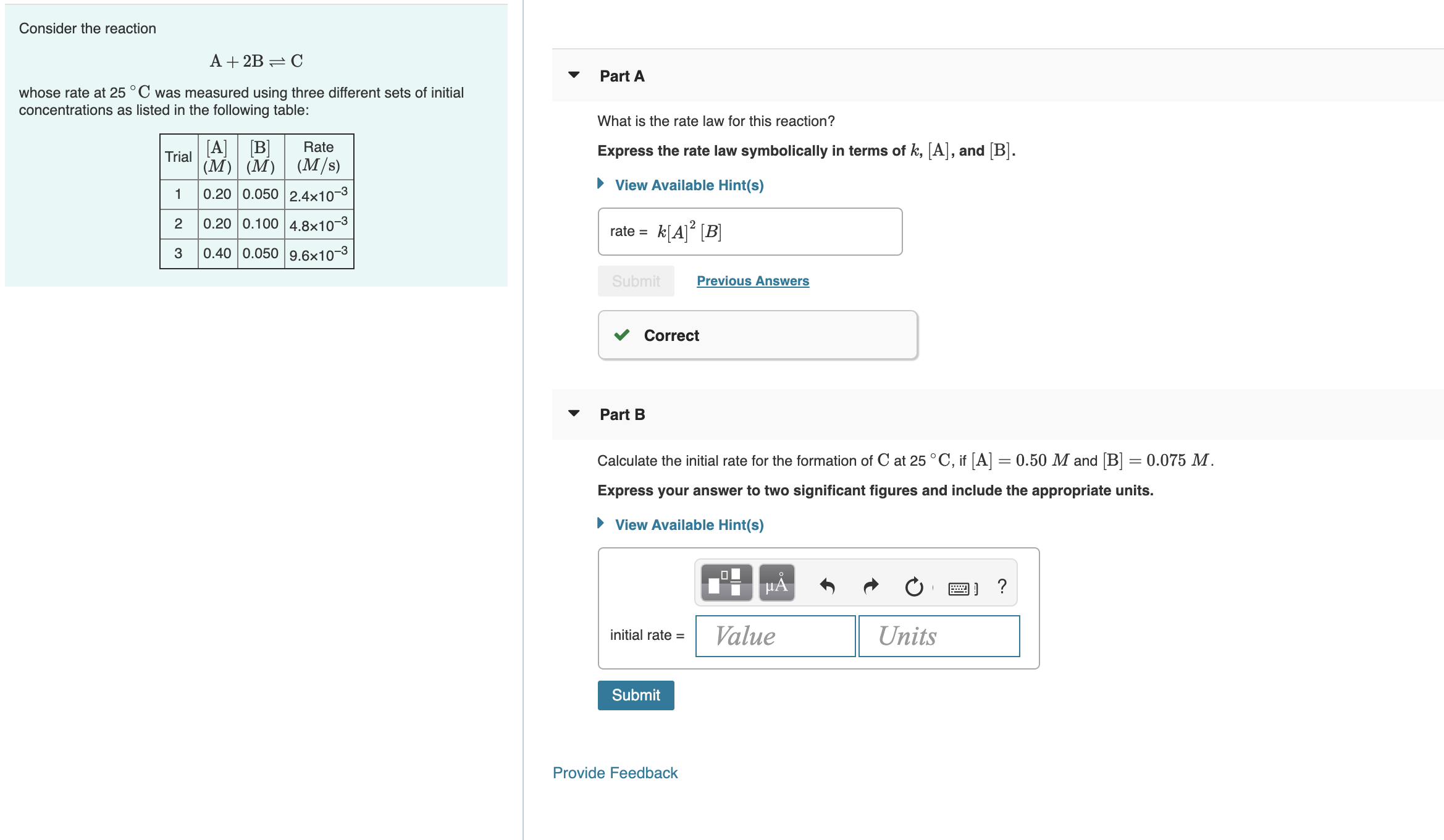
Shown below is a concentration vs. time plot for the reaction A \rightleftharpoons 2B. What is the approximate value for the equilibrium constant (Kc) for this reaction? Show your work and/or explain
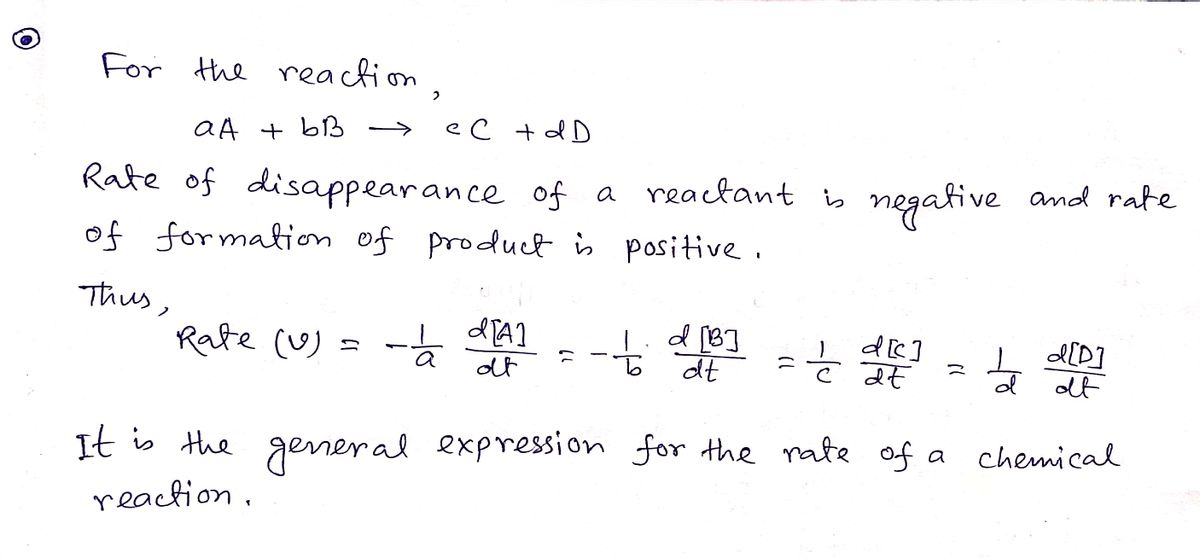
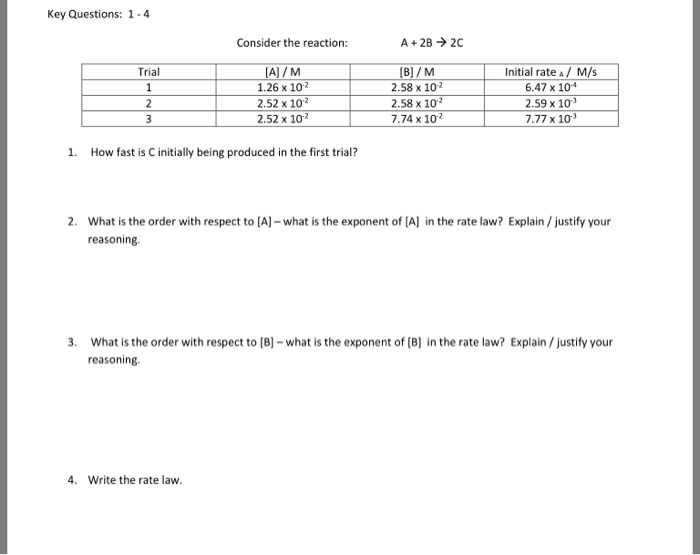
What is the order of reaction, if a reaction, A+2B—› Product, when the rate becomes doubled if the concentration of A is doubled and the rate remains the same if the concentration
![Rate of reaction, A + 2B ⟶ Productis r = k [B]^2 . If B is taken in large excess then rate of reaction is: Rate of reaction, A + 2B ⟶ Productis r = k [B]^2 . If B is taken in large excess then rate of reaction is:](https://dwes9vv9u0550.cloudfront.net/images/2793111/a9e390e5-c83e-4814-a571-e5178b34568b.jpg)
Rate of reaction, A + 2B ⟶ Productis r = k [B]^2 . If B is taken in large excess then rate of reaction is:
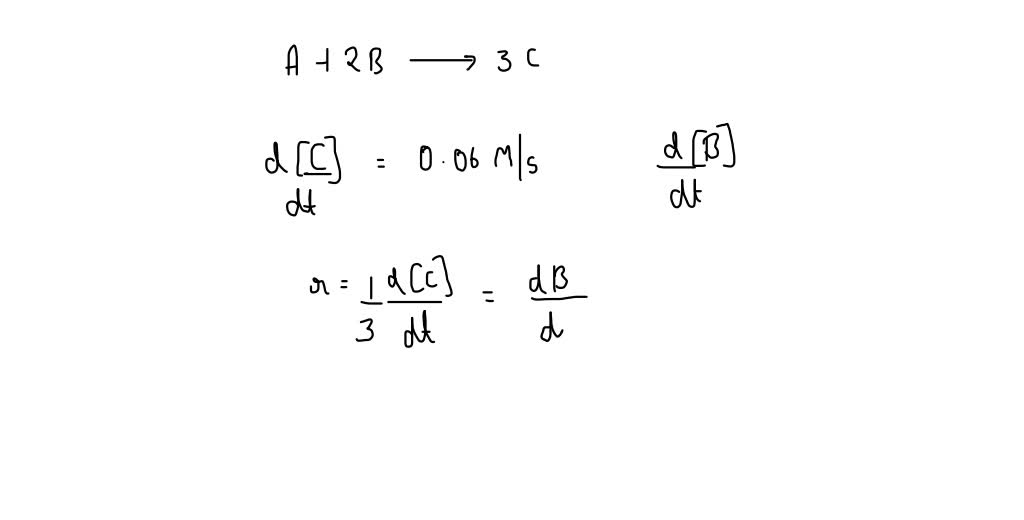

![ANSWERED] In the reaction represented by the gener... - Inorganic Chemistry ANSWERED] In the reaction represented by the gener... - Inorganic Chemistry](https://media.kunduz.com/media/sug-question/raw/52219267-1659134967.982038.jpeg)