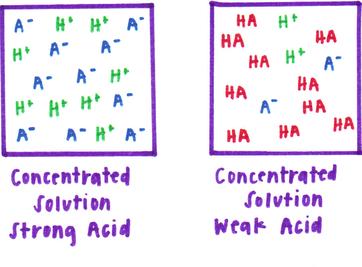
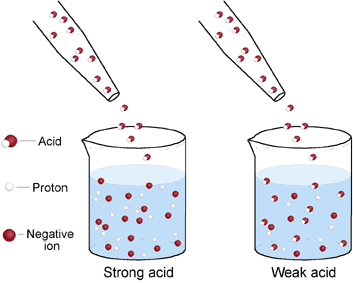
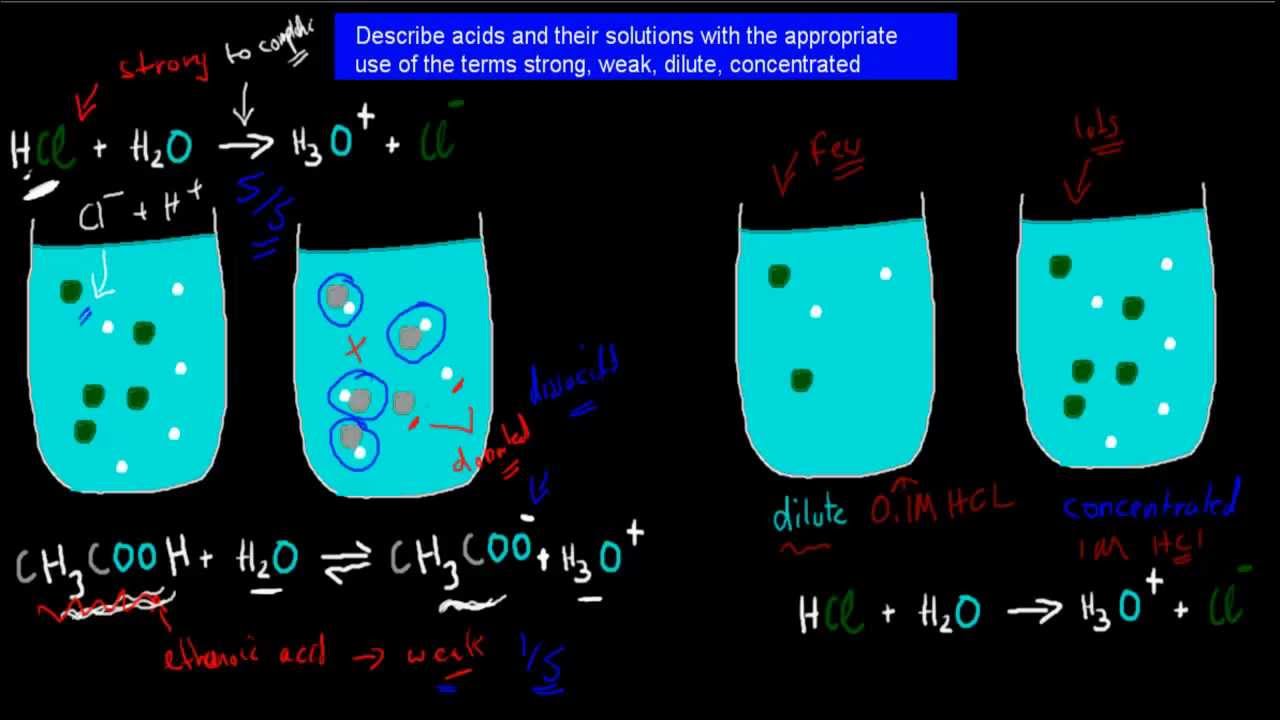
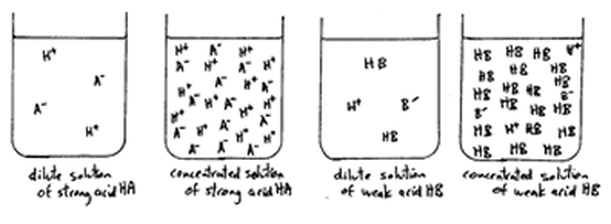
equilibrium - Does the number of H+ ions in solution go up on dilution of a weak acid? - Chemistry Stack Exchange

UNIT 4: Solutions: Dilutions & Titrations. Strong Acids An acid that ionizes completely in water is called a strong acid. Hydrochloric acid, HCl(aq), - ppt download

0.04 N` solution of a weak acid has specific conductance `4.23 xx 10^(-4) "mho cm"^(-1)`. If the - YouTube
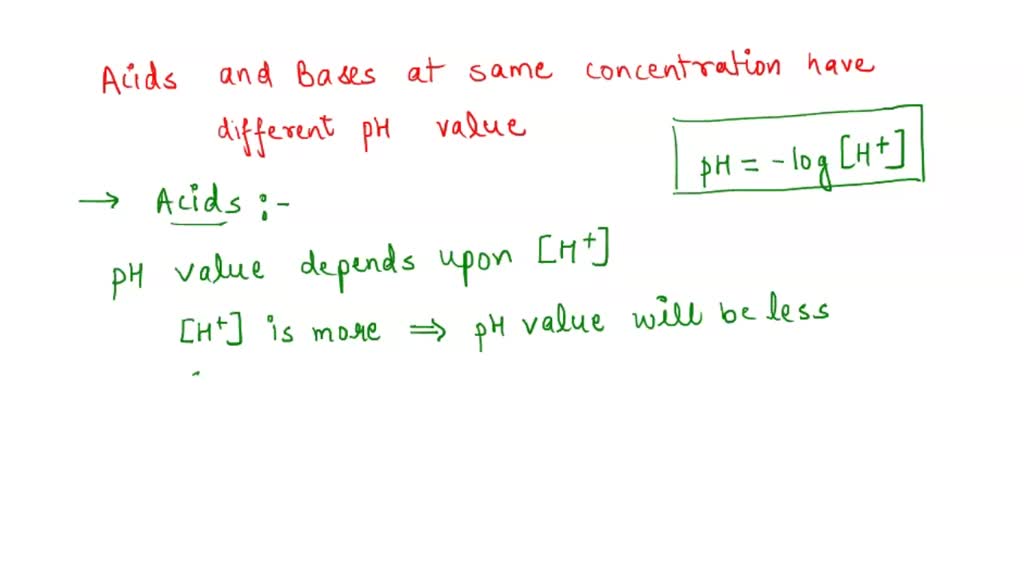
SOLVED: Assertion-On dilution of a strong acid or a weak acid the number of H+ ions per unit volume in the solution decreases. Reason-dissociation of strong acid does not increase with dilution

SOLVED: Box Wcak acid data and calculations Mcasurements Dek # Name (0) Volume of the weak acid stock solution ( diluled Concentration of the wcak acid stock solution (c) Concentration of the