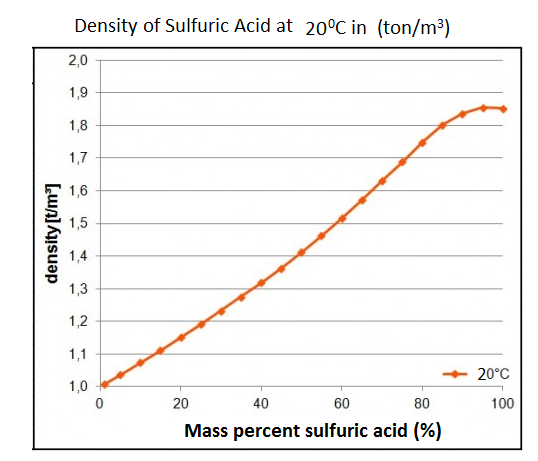
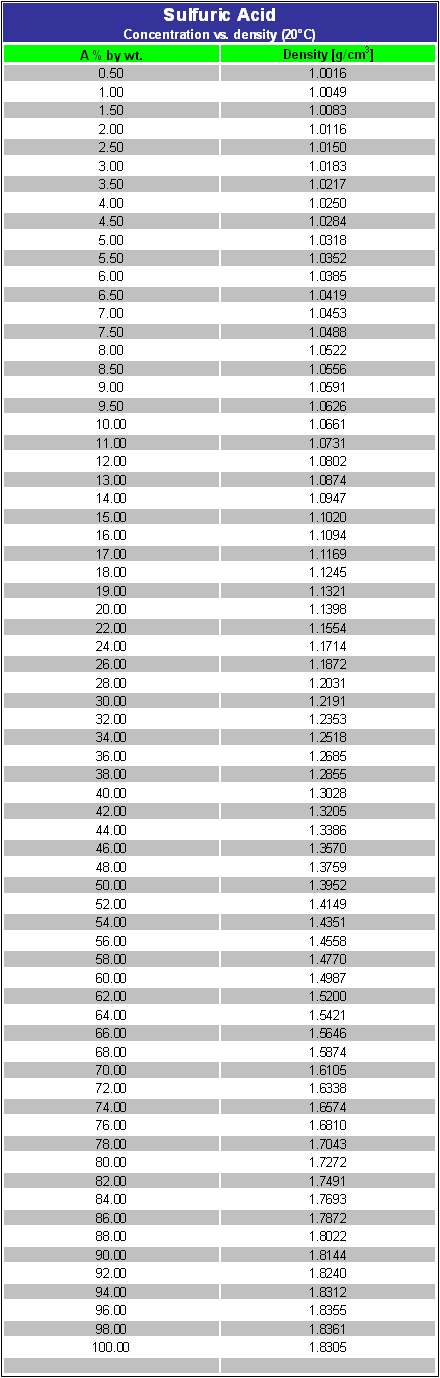
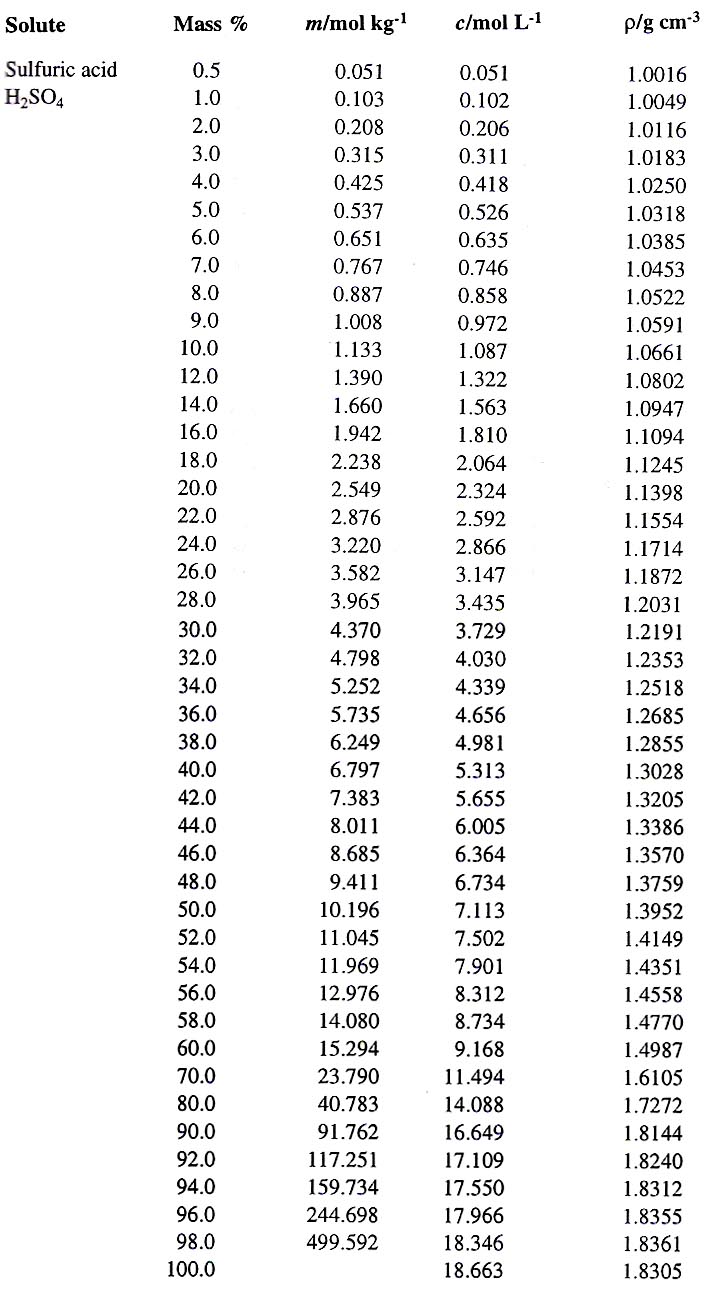
![The density (in g ml^-1 ) of a 3.60 M sulphuric acid solution having 29% H2SO4 [molar mass = 98 g mol^-1 ] by mass, will be: The density (in g ml^-1 ) of a 3.60 M sulphuric acid solution having 29% H2SO4 [molar mass = 98 g mol^-1 ] by mass, will be:](https://dwes9vv9u0550.cloudfront.net/images/5468271/2ef79989-6c93-4eae-b4f4-8ef6608caa27.jpg)
The density (in g ml^-1 ) of a 3.60 M sulphuric acid solution having 29% H2SO4 [molar mass = 98 g mol^-1 ] by mass, will be:
What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora
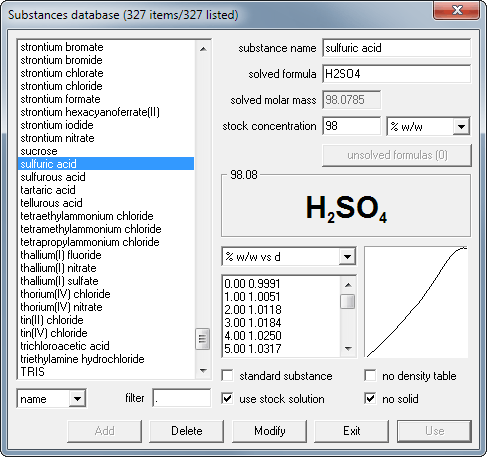
Concentration conversions using CASC - converting molarity to percent concentration, checking density

Kinetics of selenium and tellurium removal with cuprous ion from copper sulfate-sulfuric acid solution | Semantic Scholar

reactivity - How could mass increase when sulfuric acid is added to calcium carbonate? - Chemistry Stack Exchange
What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora
Chem201, Winter 2006 Name Answer key______________ Midterm N1 01/26/06 SID___________________________ 1. A solution is prepared

What volume of 95% sulphuric acid (density = 1.85 g mL^(-1)) and what mass of water must be taken to prepare 100 mL of 15% solution of sulphuric acid ( density = 1.1 g mL^(-1)) ?