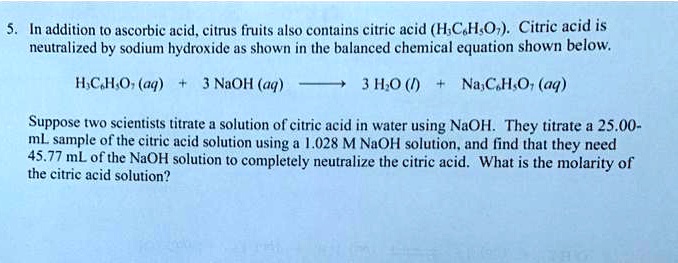
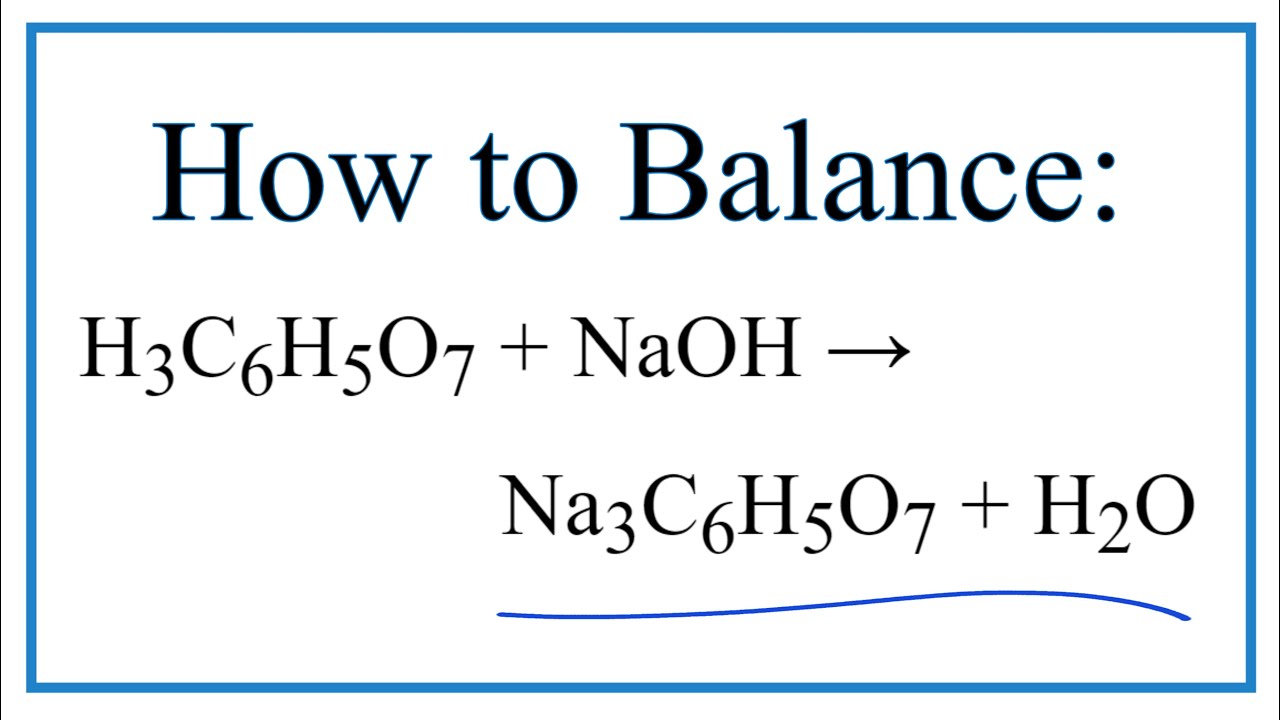
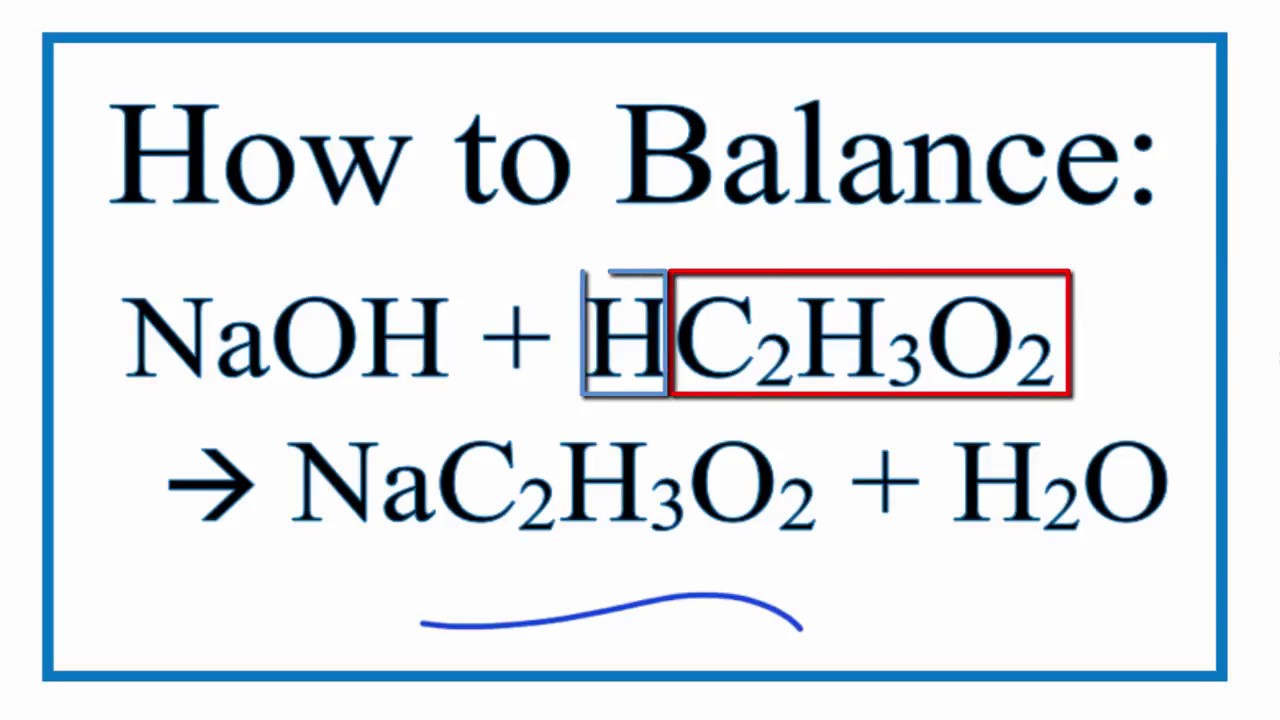
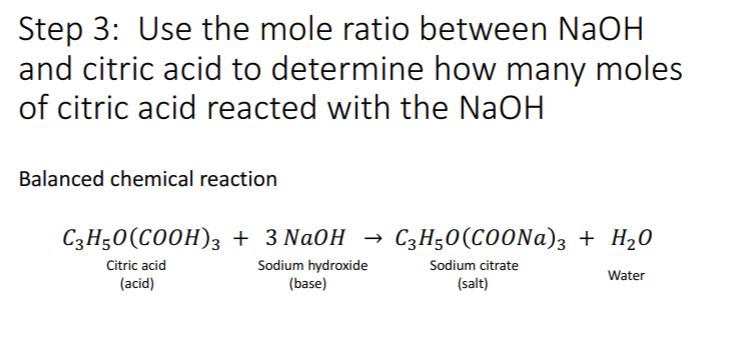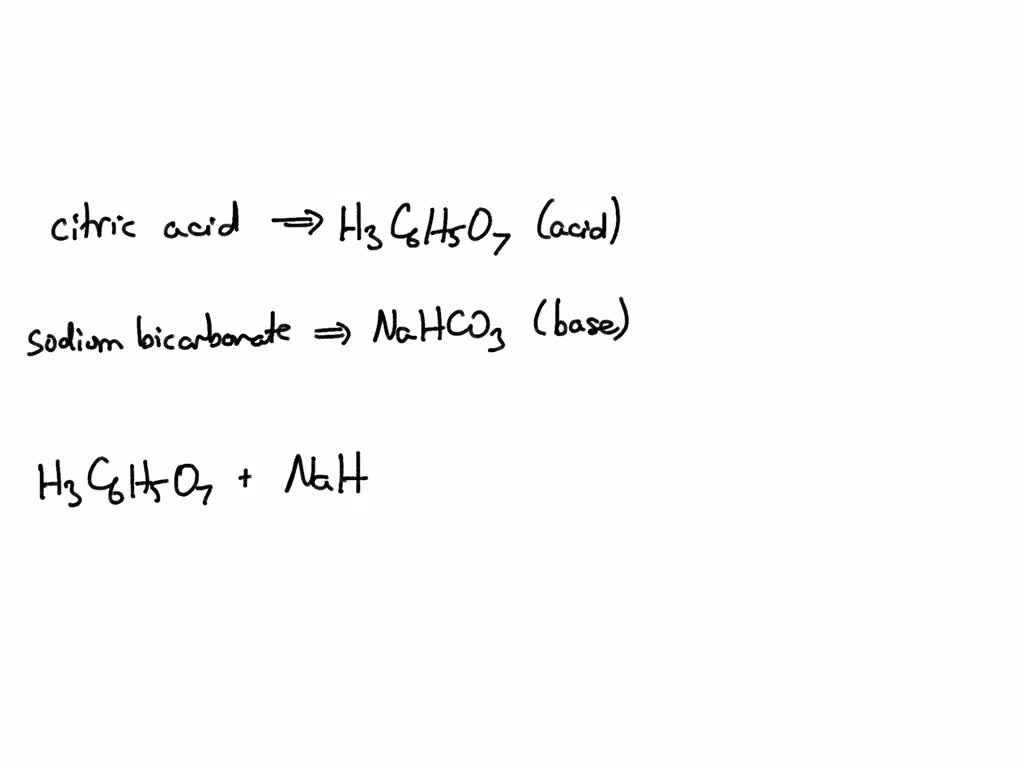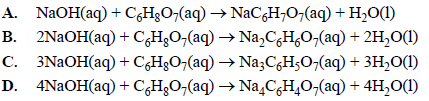OneClass: Write the balanced net ionic equation for the reaction between citric acid and sodium hydro...

OneClass: Please show ALL work. Citric acid and sodium hydroxide react with each other according ...

organic chemistry - Role of hydrochloric acid and sodium hydroxide in isolation of citric acid from lemon juice - Chemistry Stack Exchange

Buffer Solution (citric acid, sodium hydroxide, hydrogen chloride) tracable to SRM from NIST and PTB pH 2.00 (25°C) Certipur® | Sigma-Aldrich
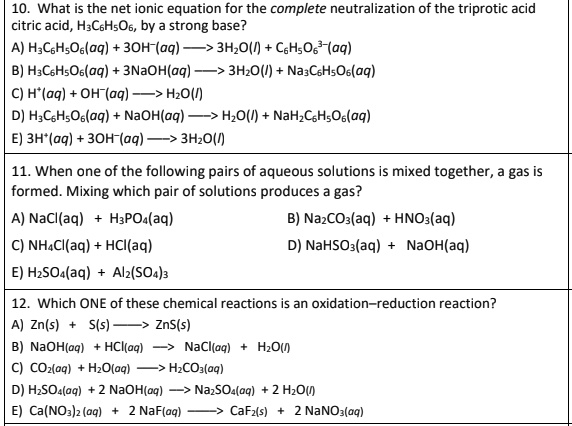
SOLVED: What is the net ionic equation for the complete neutralization of the triprotic acid citric acid, H-CbHsOb, by strong base? A) HyCsH;Os(aq) 30H-(aq) 3HzO() CsHsOs-(aq) B) H-CsHsOs(aq) 3NaOH(aq) 5s 3HzO() -