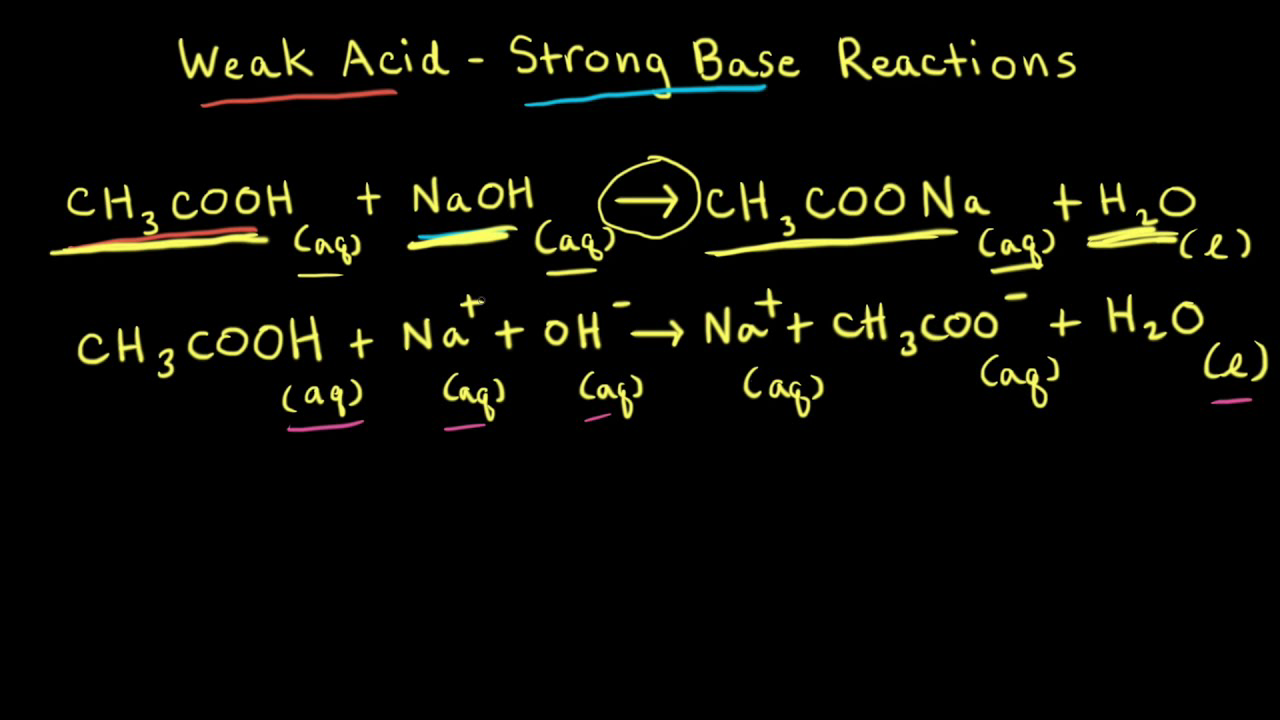Complete the below acid-base reaction and name the salt formed. (a) Et3N+HCl gives to (b) C5H11NH+CH3COOH gives to | Homework.Study.com
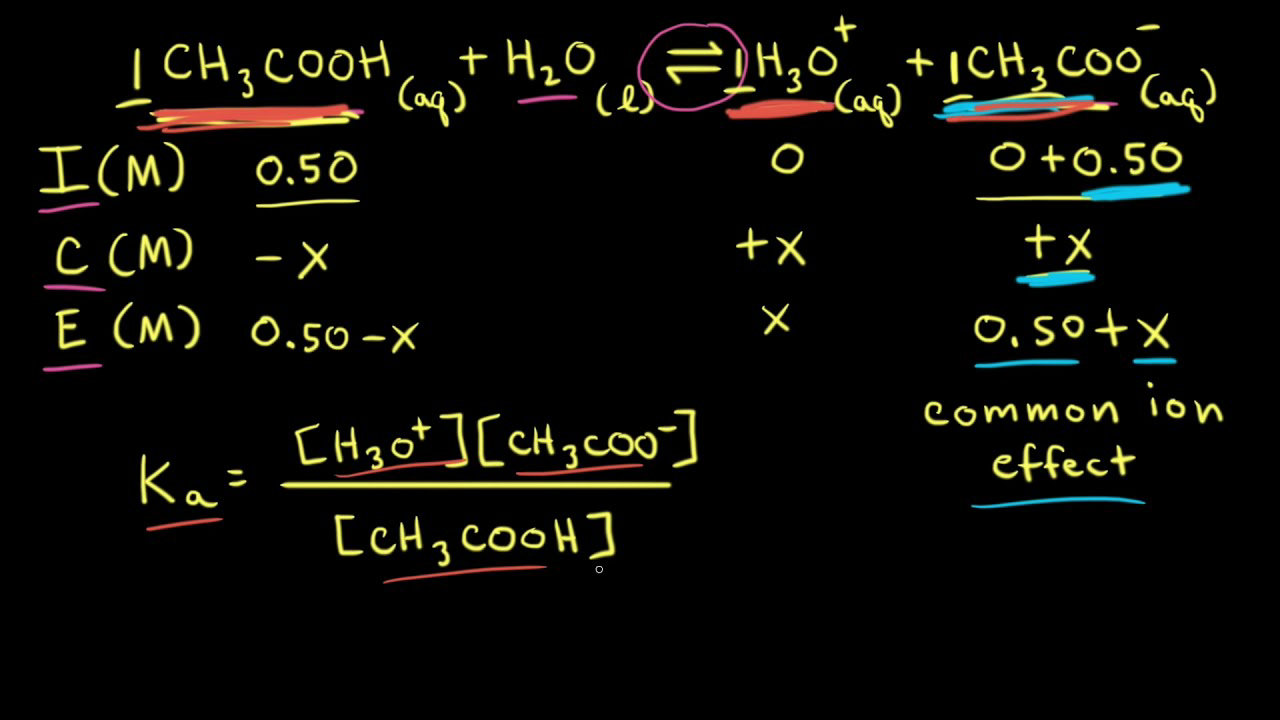
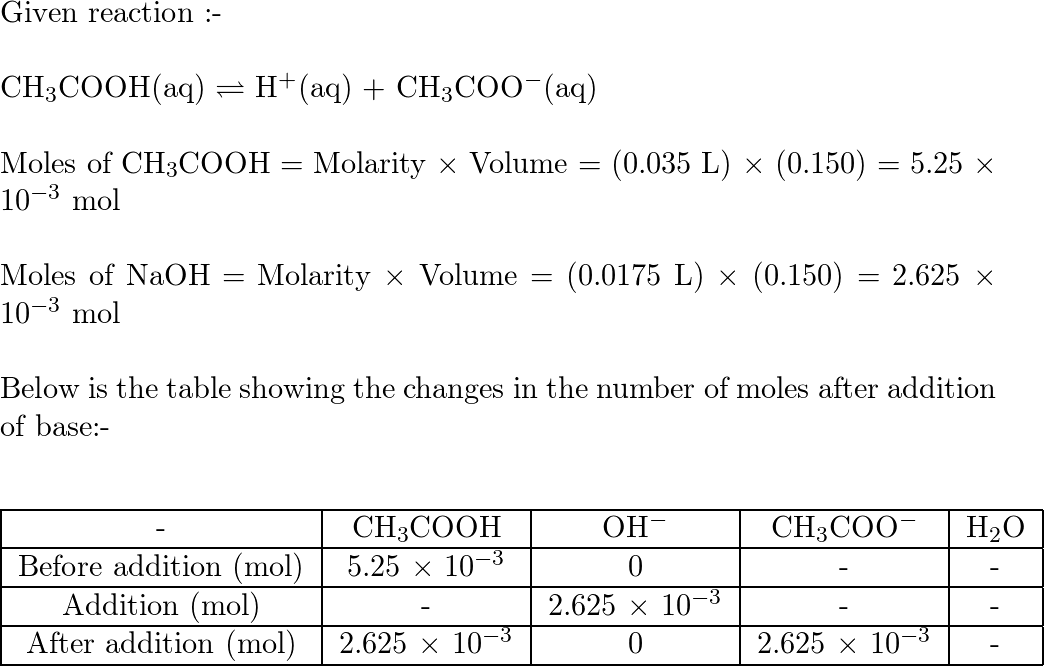
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

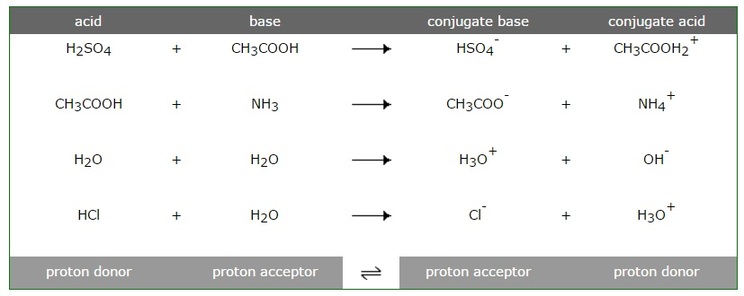
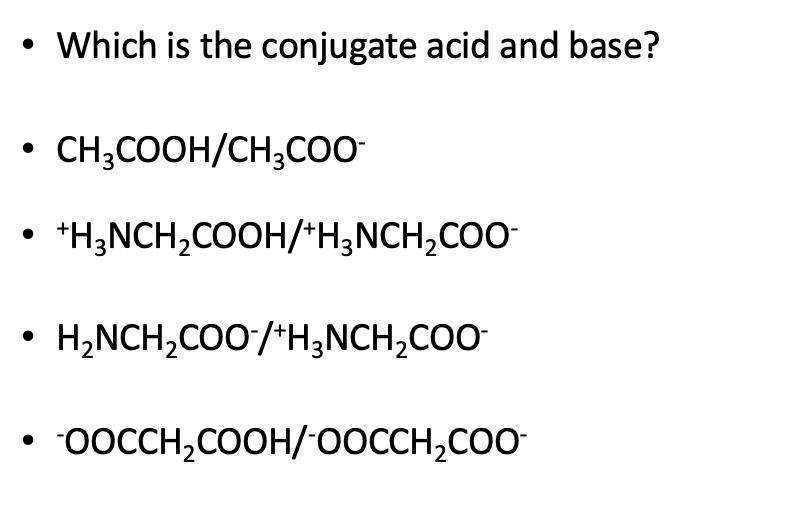
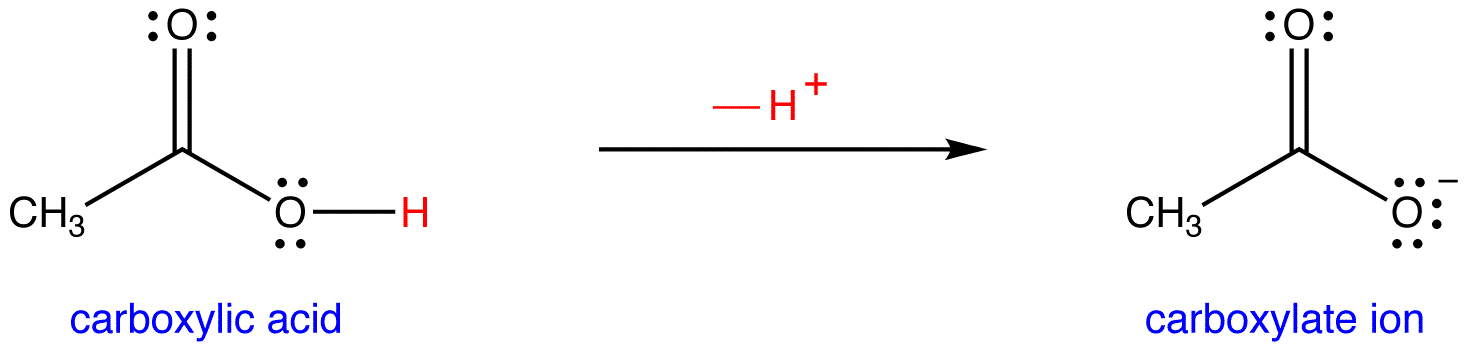
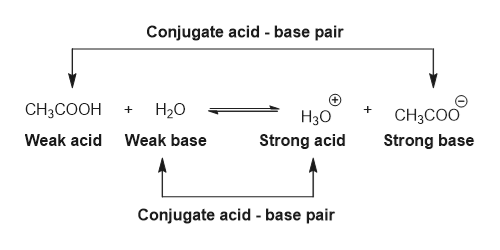
Ionic dissociation of acetic acid is represented as CH3COOH+H2OhArrH3O^(+)+ CH3COO^(-) Which of the following statement are/is correct? 1. According to Lowery and Bronstad, the reaction posses an acid and three bases. 2. H2O
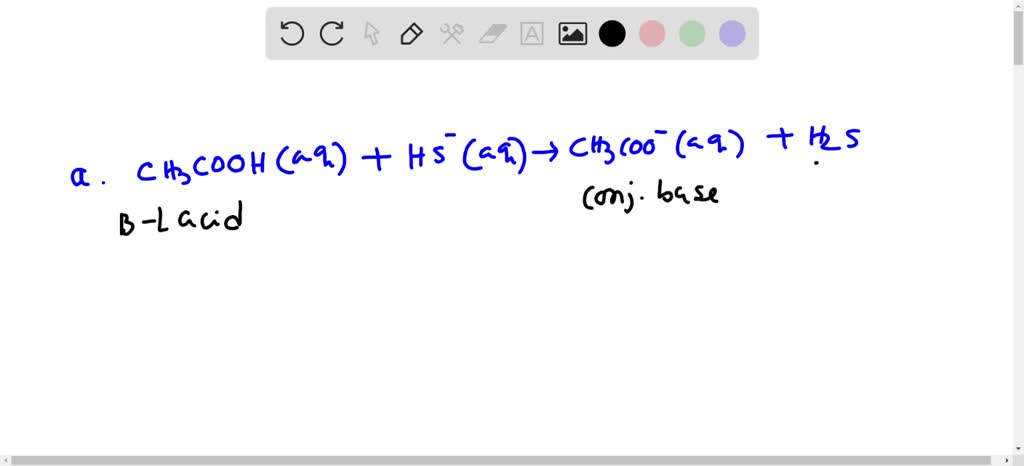
SOLVED: For each of the following reaction, identify the Bronsted-Lowry acids and bases and the conjugate acid-base pairs. a. CH3COOH(aq) + HS-(aq) —– CH3COO-(aq) + H2S(aq) b. F-(aq) + NH4+(aq) —– HF(aq) +
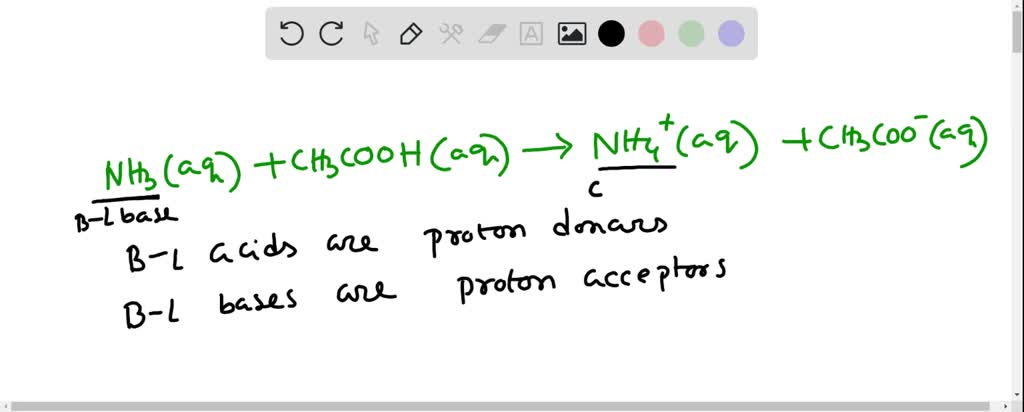
SOLVED: Identify the acid, base, conjugate acid and conjugate base in the following reactions: 1. NH3(aq) + CH3COOH(aq) —> NH4+(aq) + CH3COO- (aq)

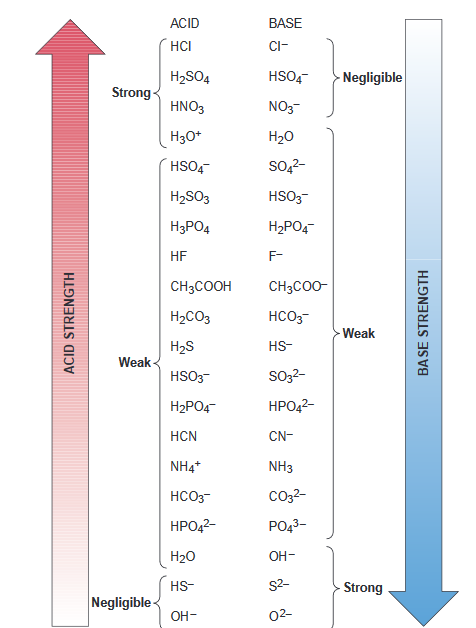
Types and Strengths of Acids and Bases in Ionic Equilibria | by Maverick Puah the Chemistry Guru | Medium