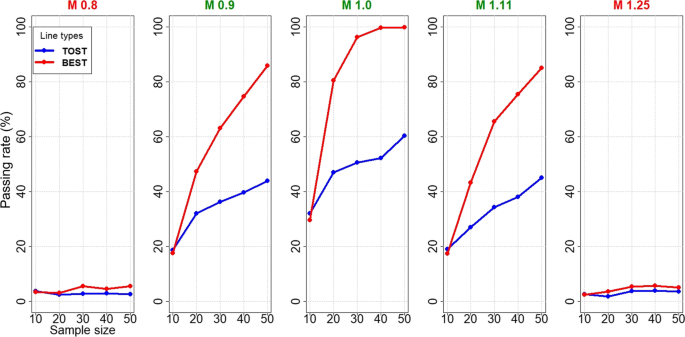
Percent of studies passing bioequivalence (BE) (power curves); average... | Download Scientific Diagram
Power curves for the determination of bioequivalence. The conditions... | Download Scientific Diagram
Applied Sciences | Free Full-Text | Machine Learning in Bioequivalence: Towards Identifying an Appropriate Measure of Absorption Rate

Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs

Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs
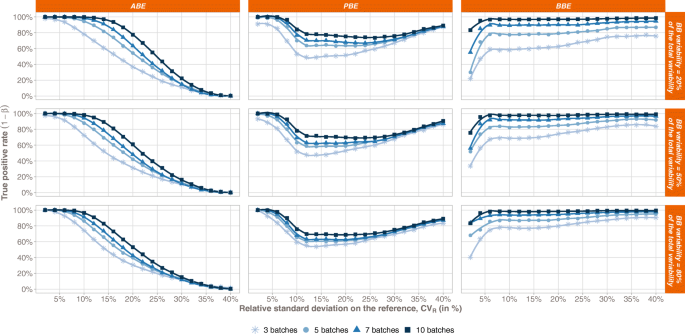
Between-Batch Bioequivalence (BBE): a Statistical Test to Evaluate In Vitro Bioequivalence Considering the Between-Batch Variability | SpringerLink

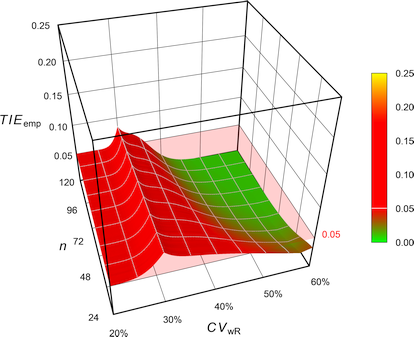
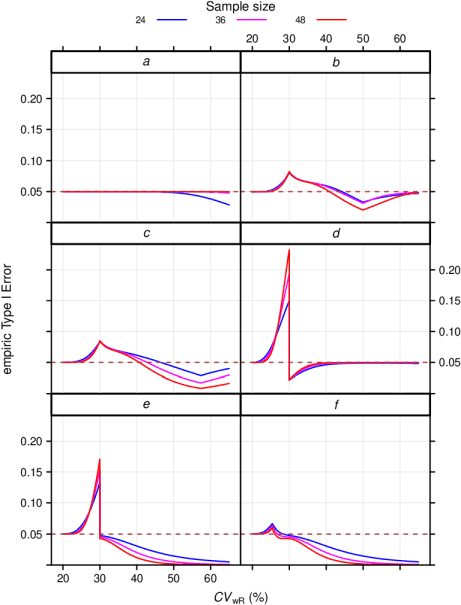
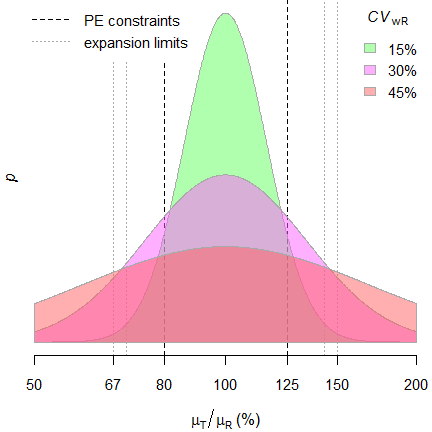
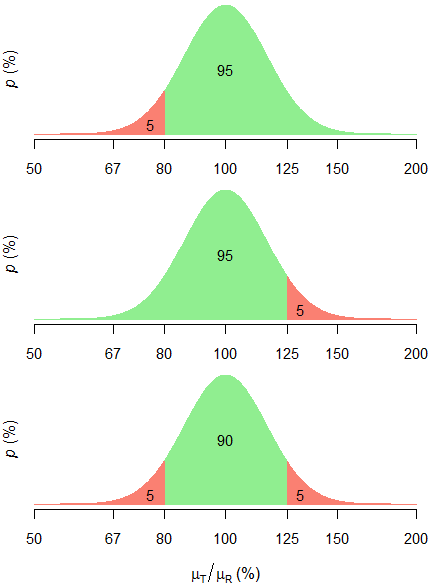
Pharmaceutics | Free Full-Text | Evaluation of a Proposed Approach for the Determination of the Bioequivalence Acceptance Range for Narrow Therapeutic Index Drugs in the European Union
Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial | PLOS Medicine
Novel Model-Integrated Design for Bioequivalence Studies of LAI Products A Complete Framework with the MonolixSuite

The 90% confidence interval for average bioequivalence measures (Cmax... | Download Scientific Diagram

Equivalence tests for ratio of means in bioequivalence studies under crossover design - Yingdong He, Yuhao Deng, Chong You, Xiao-Hua Zhou, 2022

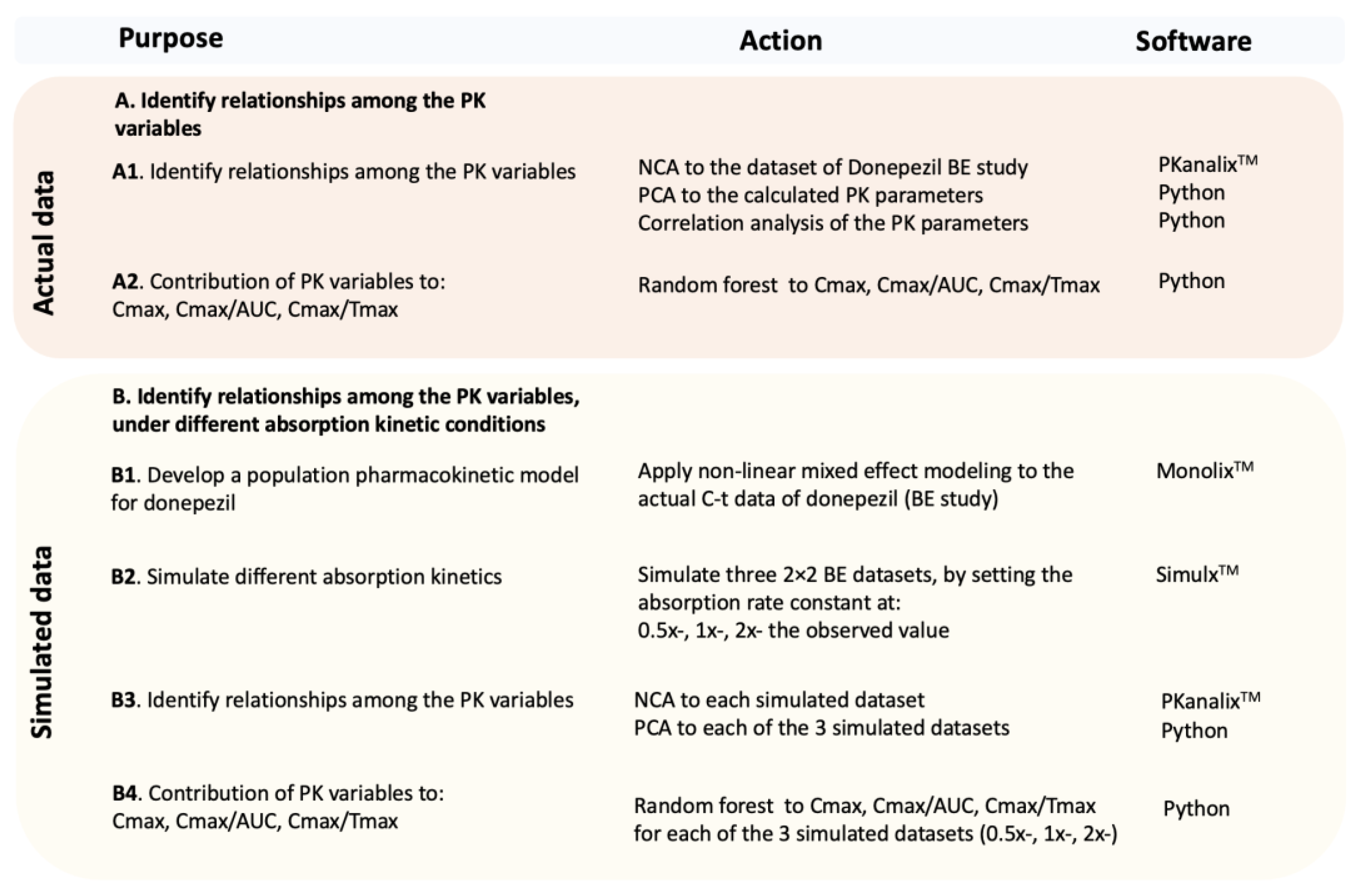
Pharmaceutics | Free Full-Text | Model-Based Equivalent Dose Optimization to Develop New Donepezil Patch Formulation

Implementation of a reference-scaled average bioequivalence approach for highly variable generic drug products of agomelatine in Chinese subjects - ScienceDirect