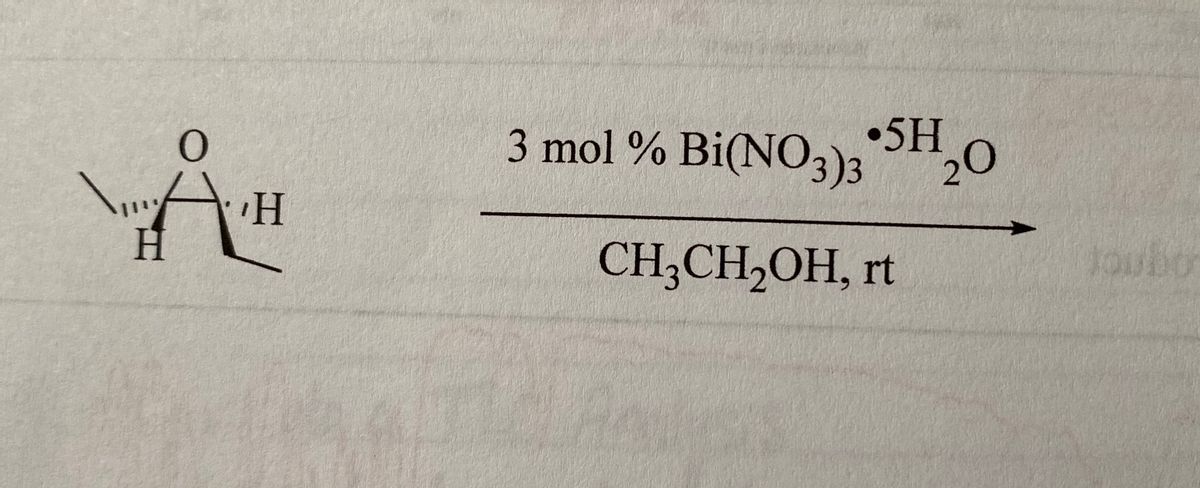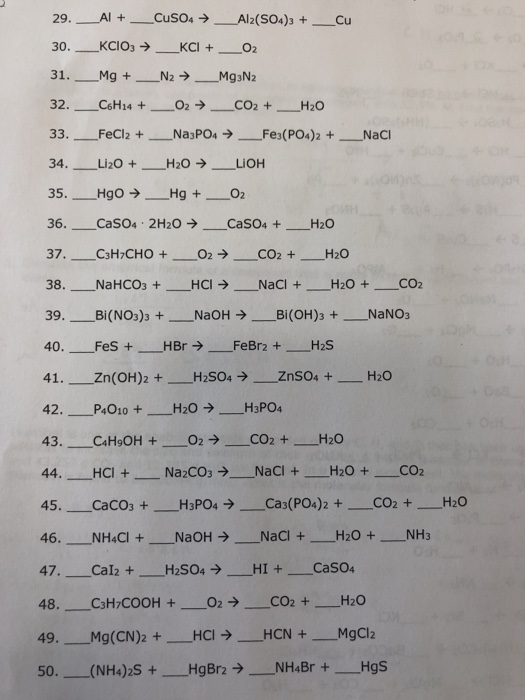Al(NO3)3 + NaOH=Al(OH)3+NaNO3 Balanced Equationaluminum- Hydroxide and Sodium Hydroxide Equation - YouTube

Controllable construction and efficient photocatalysis performance of Bi@Bi6O7FCl3 heterostructures exposed with the (012) plane bi-quantum-dots - ScienceDirect

Chapter 10 Jeopardy!!!! Can use Periodic Tables, activity series and solubility rules only!!!! (no book, no notes, etc.) Write answer LARGE & LEGIBLE on. - ppt download
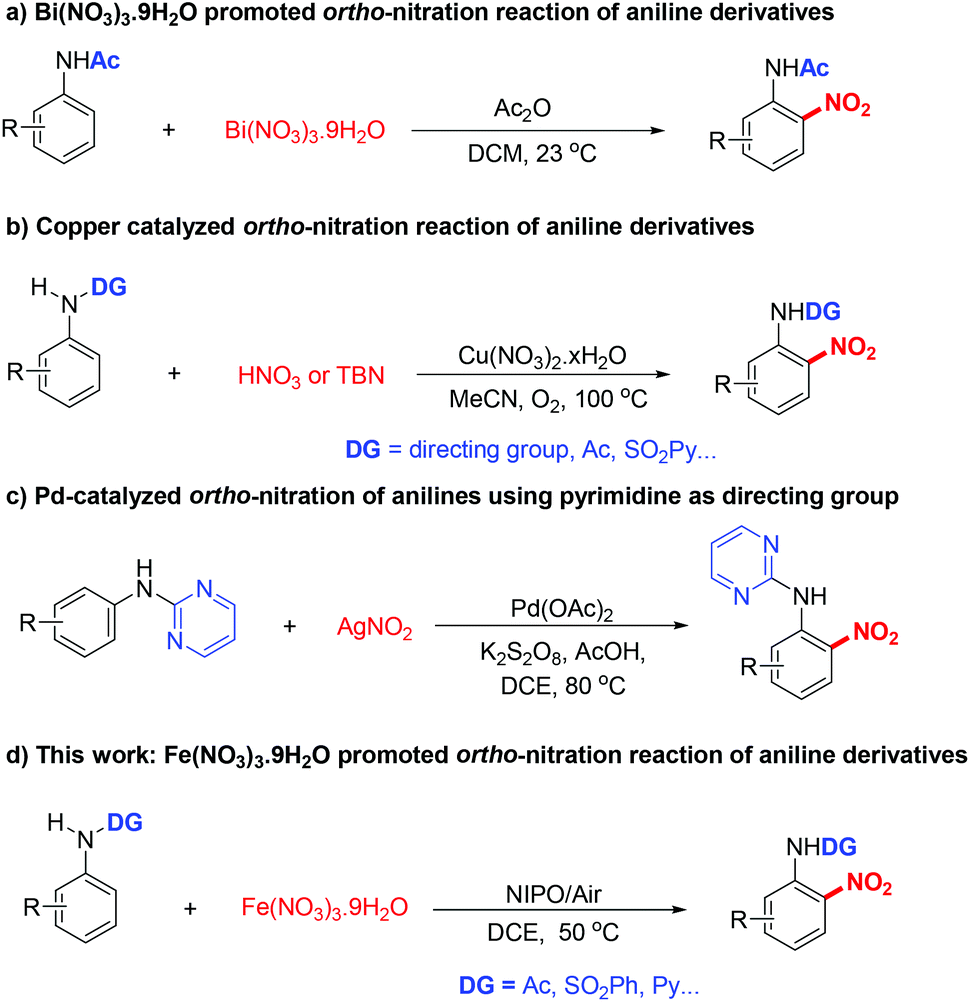
Regioselective nitration of anilines with Fe(NO 3 ) 3 ·9H 2 O as a promoter and a nitro source - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C8OB00841H

XRD patterns of the reactants (2 mmol of Bi(NO 3 ) 3 , 1.392 g of NaOH,... | Download Scientific Diagram

SOLVED: Write the net ionic equation for: 3 H2S(aq) Bi(NO3)3(aq) BizS3(s) HNO3(aq) Click here to enter oredit Your answer 3 Sz (a4)+2 Bi, (aq)-BizS , (s)
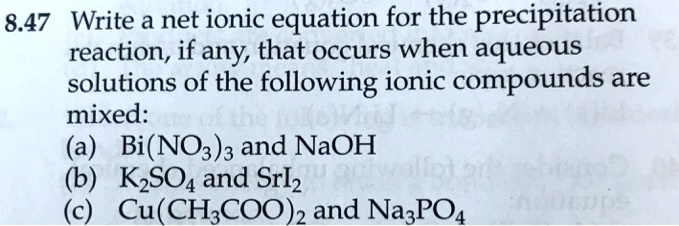
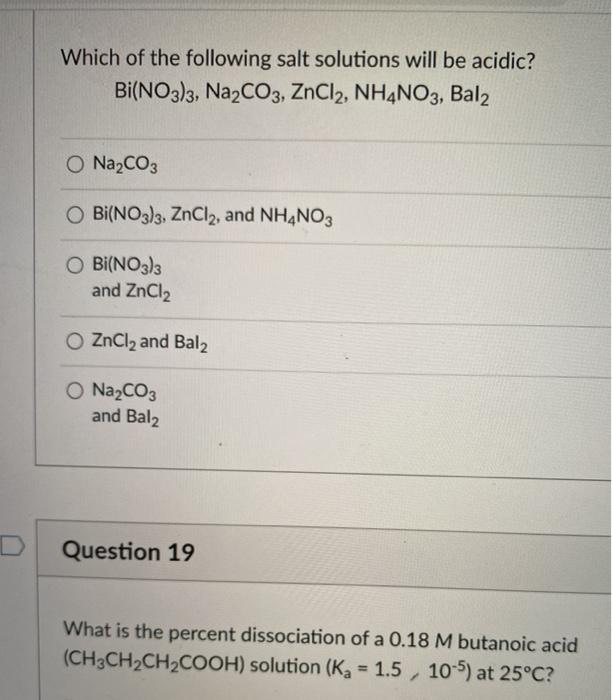
SOLVED: 8.47 Write a net ionic equation for the precipitation reaction, if any; that occurs when aqueous solutions of the following ionic compounds are mixed: (a) Bi(NO3)3 and NaOH KzSO4 and Srlz



![PDF] Hydrothermal synthesis of sodium and potassium bismuth titanates | Semantic Scholar PDF] Hydrothermal synthesis of sodium and potassium bismuth titanates | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7ab5f4365aefc6be9fd46d2c68477b872ddd589b/3-Table1-1.png)