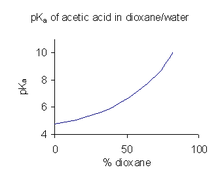
The dependency of the pKa of acetic acid on the ionic strength, at 18... | Download Scientific Diagram

Various pKa values as function of temperature. Each component has an... | Download Scientific Diagram
![SOLVED: -] PKa Of weak acids at 259C Name Formula pKal 4.756 pKaz pKa3 acetic acid CH3COzH C6HsCOzH CzH-COzH CzH6CICOzH CzHsCOzH HzC204 HzPO4 CzHsCOzH HzSO4 CaHgCOzH benzoic acid 4.204 butanoic acid 4.83 SOLVED: -] PKa Of weak acids at 259C Name Formula pKal 4.756 pKaz pKa3 acetic acid CH3COzH C6HsCOzH CzH-COzH CzH6CICOzH CzHsCOzH HzC204 HzPO4 CzHsCOzH HzSO4 CaHgCOzH benzoic acid 4.204 butanoic acid 4.83](https://cdn.numerade.com/ask_images/b298d530b31c4de89c3a7162e4a956e1.jpg)
SOLVED: -] PKa Of weak acids at 259C Name Formula pKal 4.756 pKaz pKa3 acetic acid CH3COzH C6HsCOzH CzH-COzH CzH6CICOzH CzHsCOzH HzC204 HzPO4 CzHsCOzH HzSO4 CaHgCOzH benzoic acid 4.204 butanoic acid 4.83

pK(a) value for acetic acid at an experimental temperature is 5. The percentage hydrolysis of 0.1 M sodium acetate solution will be
Can temperature affect the concentration of acetic acid in vinegar without evaporation and condensation being a factor? - Quora

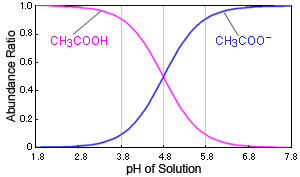
SOLVED: Given the pKa of acetic acid IS 4.74 , calculate the pH of a butfer solution that contains 0.80 M of acetic acid (CH,COOH) and 0.20 M 0f sodium acetate (CH

Plots of Λ versus √c required for pKa determination of formic acid at... | Download Scientific Diagram

The dependency of the pKa of acetic acid on the ionic strength, at 18... | Download Scientific Diagram

PDF) Dissociation Constant of Acetic Acid in (N,N-Dimethylformamide + Water) Mixtures at the Temperature 298.15 K

Acid Dissociation Constant (pKa) of Common Monoethylene Glycol (MEG) Regeneration Organic Acids and Methyldiethanolamine at Varying MEG Concentration, Temperature, and Ionic Strength | Journal of Chemical & Engineering Data

The degree of dissociation of acetic acid in a 0.1 M solution is `1.32xx10^(-2)`, find out the pKa : - YouTube
Acid Dissociation Constant (pKa) of Common Monoethylene Glycol (MEG) Regeneration Organic Acids and Methyldiethanolamine at Vary