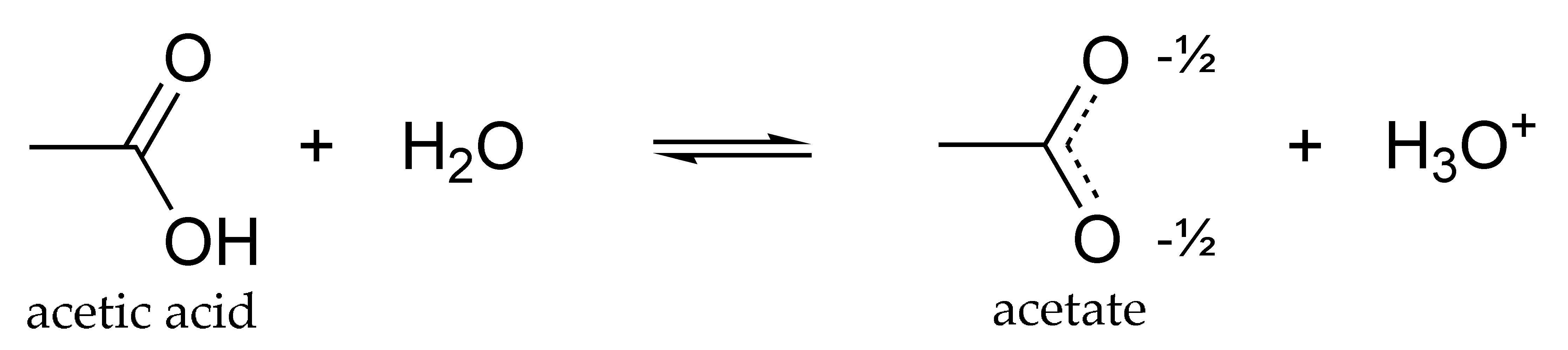Why does the solution of sodium acetate give more concentration of Hydroxide ion? Shouldn't the number of Hydroxide ion and hydrogen ion be equal? - Quora

pKa for acetic acid is 4.74 . What should be the ratio of concentration of acetic acid and acetate ions to have a solution with pH 5.74 ?

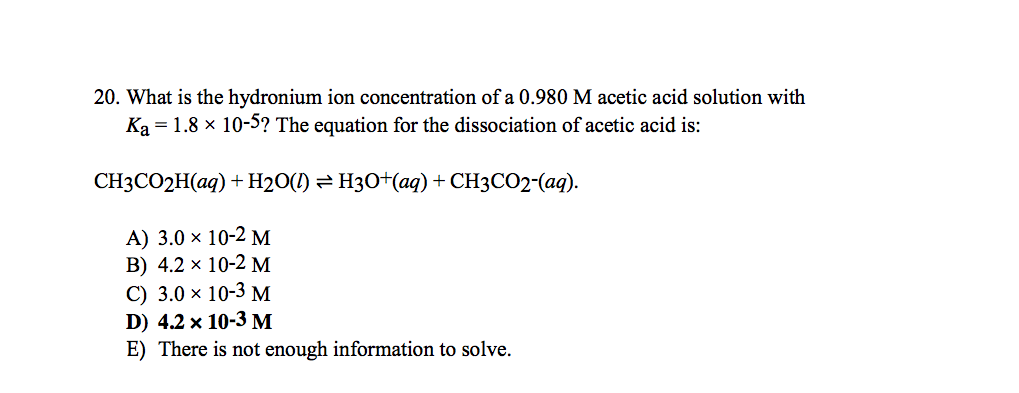
SOLVED: Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aquesous solution: HCzH3Ozlaq) = CzH3Oz (aq) + Ht(aq) At equilibrium at 259C a 0.102M solution

Aqueous Equilibria The Common-Ion Effect Consider a solution of acetic acid: NaC 2 H 3 O 2 Le Châtelier says the equilibrium will shift to the ______. - ppt download

What is the pH of the solution when 0.20 mol of HCI is added to 1L of a solution containing a. 1M each of acetic acid and acetate ion. b. 0.1Meach of

SOLVED: What Is the complete ionic, net ionic equation and the spectator ion for the acid- base reaction that occurs when acetic acid and potassium hydroxide solutions are mixed? (4 Points) (d)CH;COO- (

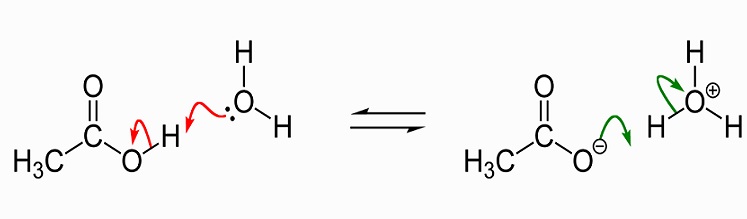
In terms of the bonds present, explain why acetic acid, CH_3CO_2H, contains two distinct types of carbon-oxygen bonds, whereas the acetate ion, formed by loss of a hydrogen ion from acetic acid,