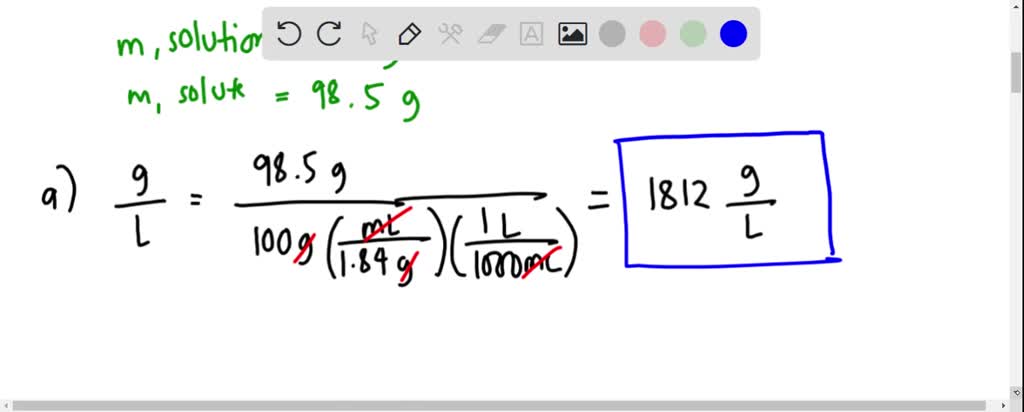
What is the volume of concentrated H2SO4 of specific gravity 1.84 and containing 98% H2SO4 by weights that would contain 40 gm of pure H2SO4? - Quora

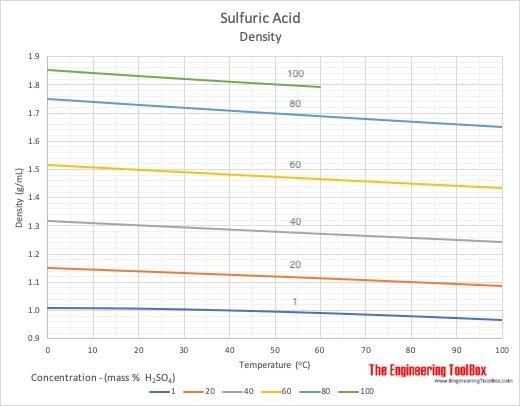
Concentrated aqueous sulphuric acid is `98% H_(2)SO_(4)` by mass and has a density of `1.80 g mL - YouTube

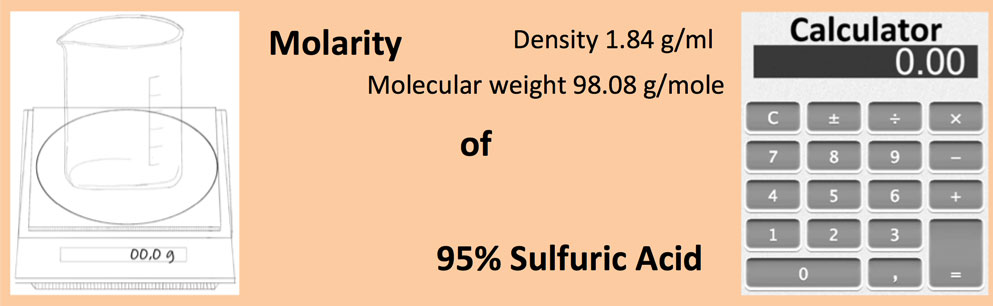
What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora

What volume of 96% H2SO4 solution (density 1.83 g/mL) is required to prepare 4 litre of 3.0 M H2SO4 solution?
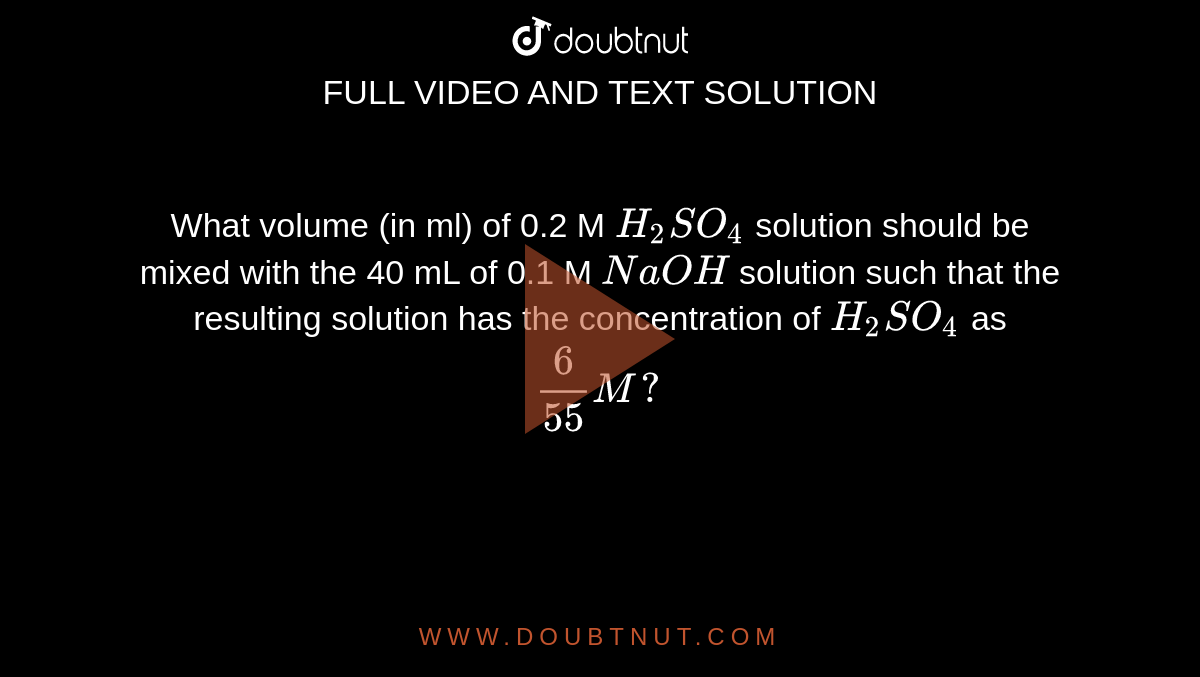
a) What is the normality of a 96 per cent solution of H(2)SO(4) of specific gravity 1.84 ? (b) How many mL of 96 per cent sulphuric acid solution is necessary to
IBP1218_09 CORROSION BY CONCENTRATED SULFURIC ACID IN CARBON STEEL PIPES AND TANKS – STATE OF THE ART Zehbour Panossian , Neus

What is the molarity of concentrated sulfuric acid if it is 96% by mass H2so4 and has a density of 1.84g/mL? - Quora
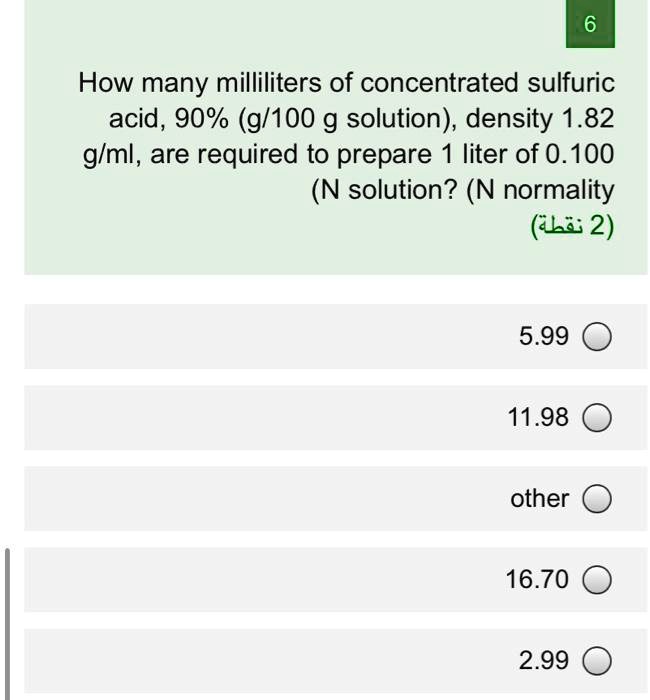
SOLVED: How many milliliters of concentrated sulfuric acid, 90% (g/100 g solution), density 1.82 glml, are required to prepare liter of 0.100 (N solution? (N normality (LE 2) 5.99 11.98 other 16.70 2.99

Sulfuric Acid, 96% Solution in Water, Extra Pure, Thermo Scientific Chemicals, Quantity: 1L | Fisher Scientific